Physics AS
Introduction Physical Quantities & Base units Vectors & Scalars Kinematics Pressure & Density Dynamics Momentum Energy, power & efficiency Deformation of solids Waves Superposition Stationary waves Electric fields Electricity Circuits & Kirchoff's laws Radioactive Physics AS PracticalPaper 5More
Reference Pastpaper QuestionsPressure, density & mass
"It's not about how bad you want it, it's about how hard you are willing to work for it." - an advice for your exams
Before, you get know what is density and pressure. We need to know what is mass
The formal definition of mass is the amount of inertia or resistant to change in motion. A less informal definition is the amount of matter or substance an object has
Density
The mass per unit volume
SI units: kgm-3
Density = Mass/Volume
D = m/v
Sometimes they give density in gcm-3 So remember this:
1gcm-3 = 1000kgm-3
Pressure
It is the force acting on per unit area
SI units: Pascals or Nm-2
Remember that 1 atm = 105Pa
Pressure in Liquids
Pressure in liquids act in all directions and it increases with depth and doesn't depend on the shape of the container
Pressureliquid = Density * g * depth
P = dgh
The derivation is sometimes asked in Mcqs

P = hdg
So where is pressure examples used in:
- Boyles law - A2
- Manometer
- Barometer
This is covered in A levels
So this actually measures the pressure difference. The difference in height tells us the difference in pressure in term of hdg. In other words, the pressure at one end is greater than the other end by hdg
So just remember this formula: These questions are usually confusing so watch this example to understand this fully Usually when the pressure difference halves the difference in height also halves. So on one side, the level decreases by 1/4 only. Similar to a manometer but, it is used to measure the atmospheric pressure. The height of mercury liquid provides a pressure which counteracts the pressure exerted by the atmosphere. So when the atmospheric pressure is equal to the mercury column pressure, we can find the pressure by using hdg to find the liquid pressure exerted by the mercury column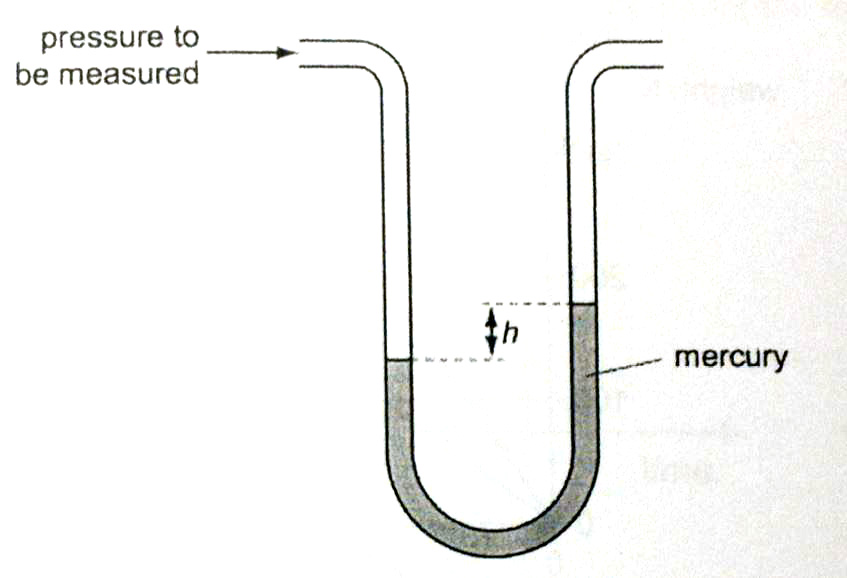
Pgas= hdg + atm
Patm = Density of Mercury * Height * g
Recommended
These are things you might like. Clicking these ads can help us improve our free services in the future...
End of Chapter Videos
Collection of Videos to Support Your Understanding.
Remember these videos are handpicked by me and I feel these are the best ones out there. But I constantly update this list for each chapter. The Youtubers are more than welcome to contact me.
Also, don't forget to Subscribe to our Youtube channel - MrWik
Watch
