Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsAlcohols & Carboxylic acids
The alcohols have an OH group attached to the carbon atom
Alcohols are very reactive and there are a number of reactions it can get involved in
Structure of Alcohols
The structure of an alcohol is very important especially when an alcohol oxidises. The structure of the alcohol determines the product formed. This is similar to structures of carbocations, there are 3 types of alcohols:
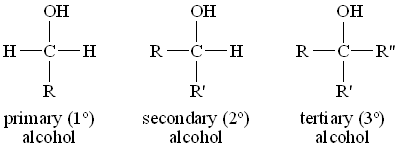
The Primary alcohol has a single R groups and 2 hydrogen atoms. This is considered to be the least stable
The Secondary alcohol has two R groups and 1 hydrogen atom
The Tertiary alcohol has 3 R groups attached. This is considered to be the most stable
An R group can be either an alky group or a long chain of carbon atoms
Remember that we only focus on the carbon atom that is bonded to the OH group. In the case of carbocations, we considered the carbon atom with the positive charge
Different types of alcohols oxidises in different ways. This might be obvious as oxidation must occur at the OH region but either alcohol will produce a carbonyl compound - A ketone or an aldehyde / Carboxylic acid
Reactions of Alcohol
There is incomplete and Complete combustion. You will need to know the products for each reaction so we will take ethanol as the example
When Ethanol react in excess oxygen (Complete Combustion):
C2H5OH + 3O2 → 2CO2 + 3H2O
When Ethanol is burnt in limited oxygen, it has several different equations:
C2H5OH + 2O2 → 2CO+ 3H2O
C2H5OH + O2 → 2C+ 3H2O
The reason why I have given two equations is that sometimes the questions may ask the production of a black substance only which is carbon ( or soot )
However, alcohol react with a cleaner flame than alkenes or alkanes but releases less energy compared to them. Alcohols are renewable fuels and more enviromentally friendly. This is because, more trees have to be grown to produce more alcohol which takes in the carbon dioxide produced from burning ethanol
During this reaction the OH group is replaced by a Chlorine atom and forms an halogenoalkane. It also produces white fumes which is HCl gas and it also releases SO2 or POCl3
C2H5OH + SOCl2 → SO2 + C2H5Cl + HCl
C2H5OH + PCl5 → POCl3 + C2H5Cl + HCl
This is also used as a test for the presence of the OH group
Alcohol is not an acid but it can lose its hydrogen atom when reacting with sodium metal. The reaction is very similar to when we add sodium metal to water:
2Na + 2C2H5OH → 2C2H5ONa + H2
We need to understand that this happens for any OH group and this is only for sodium metal. If it was a sodium ion or any other thing then it will not react. Also the rate of reaction decreases when we use larger alcohols
When alcohol vapour is passed through heated aluminium oxide (pot clay) or silicon dioxide then the alcohol is broken down to the corresponding alkene
C2H5OH → C2H4 + H2O
This requires a high temperature and also aluminium oxide as the catalyst
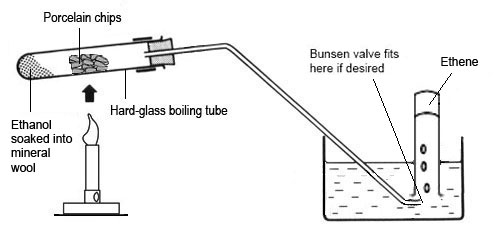
However, the same thing can be achieved by adding concentrated sulfuric acid as this will act as a powerful dehydrating agent
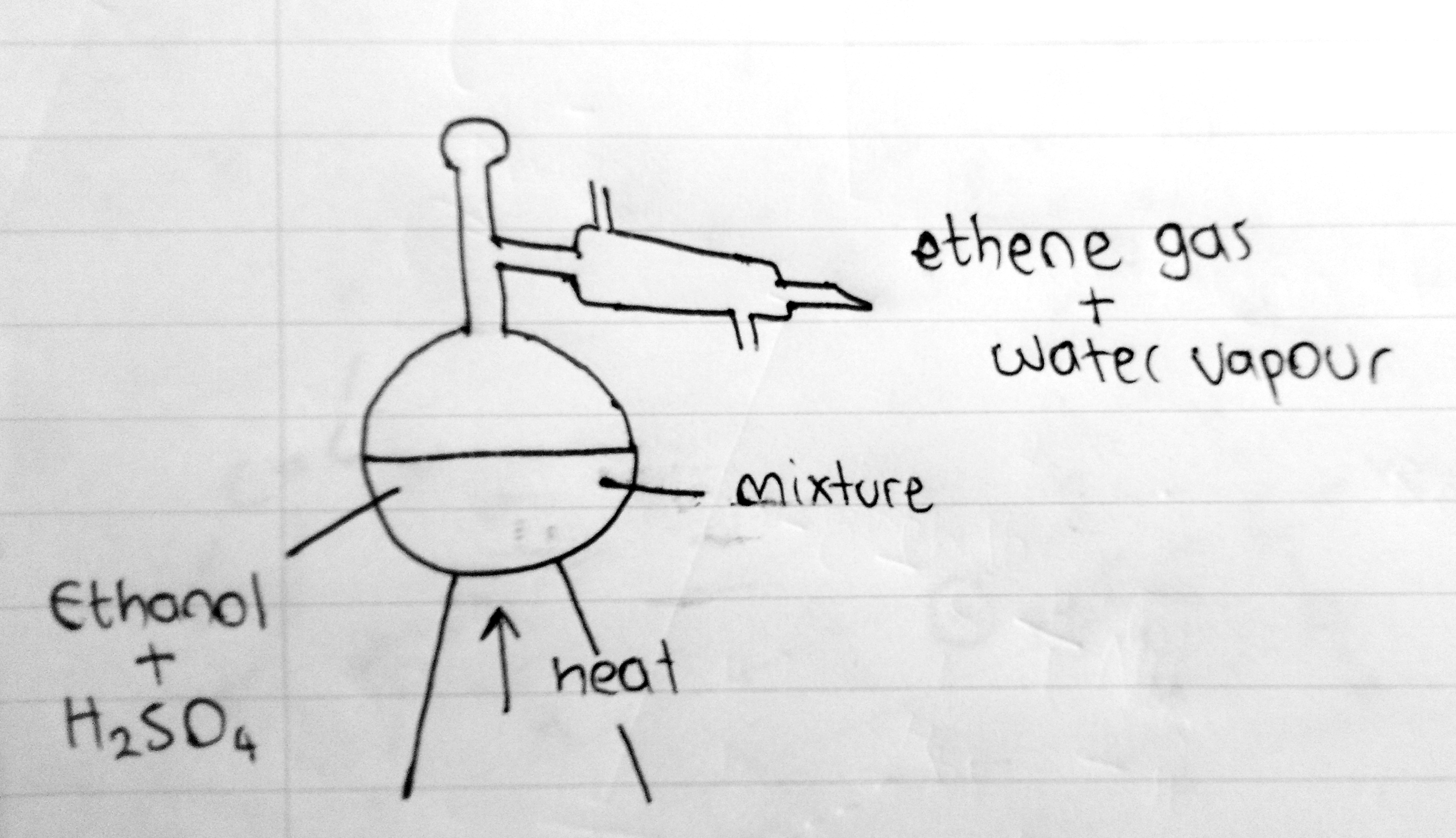
What really happens here is that the Hydrogen and the OH group breaks off and is released as a water molecule. And the point where the atoms are removed the double bond is formed
Alcohols will not react with halogens but will react with hydrogen halides as they are polar molecules
It is very similar to the reaction of ethene and hydrogen halide
C2H5OH + HCl → C2H5Cl + H2O
When an alcohol is heated with acidified Potassium Dichromate (K2Cr2O7). Then we can get either an aldehyde or carboxylic acid or a ketone
Primary alcohols are oxidised to aldehydes and can be further oxidised to carboxylic acids. Usually we write the end product as carboxylic acids but, it can depend. Let us take C2H5OH as the example of a primary alcohol
C2H5OH + [O] → CH3CHO + H2O
This is known as partial oxidation of primary alcohols which means only a few drops of dilute acidified Potassium Dichromate (K2Cr2O7) is added and it is not heated under reflux but collected as soon as possible
However, if this was heated under reflux with concentrated acidified Potassium Dichromate (K2Cr2O7) then we will get a carboxylic acid
C2H5OH + [O] → CH3COOH + H2O
This is because the Aldehyde formed reacts and further oxidises to form carboxylic acid
When secondary alcohols oxidises it can only have one possibility and that is a ketone. Ketones can not be further oxidised to any other product. So we usually heat the secondary alcohol under reflux in Concentrated acidified K2Cr2O7.
We will take propan-2-ol as the example:
CH3CH(OH)CH3 + [O] → CH3COCH3 + H2O
So in this reaction a ketone functional group can be produced as there are carbon atoms on both sides
Teritary alcohols will not oxidise or react with potassium dichromate under any conditions. In fact this could be used to differentiate between primary alcohols and teritary alcohols or secondary alcohols with tertiary alcohols. When the alcohol gets oxidised by K2Cr2O7 then the potassium dichromate ions reduce. This causes a color change from yelow/orange to green
| Alcohol | Color Change of K2Cr2O7 |
|---|---|
| Primary | Yellow to green Quickly |
| Secondary | Yellow to green Slowly |
| Teritary | Remains Yellow/orange |
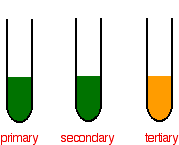
When alcohols react with carboxylic acids in the presence of concentrated sulfuric acid, then we will get an ester
Let us see an example:
CH3COOH + C2H5OH → C2H5O2CCH3 + H2O
The reaction occurs at the two group ends. An H from the COOH and a OH from the alcohol is removed to produce a water and a larger chain called an ester
The naming syntax is very easy and it goes like this:
Alcohoyl Carboyxlate
In this case, it is a ethylethanoate. The real problem is identify the name using the given structure only. Actually it is very easy and there is a simple method to identify which one is the carboxylic acid part and the alcohol part. First, look for the CO2 and remember that this was the COOH group before so this part must be the carboxylic acid. For example, C2H5O2CCH3 is an ester and we can see the O2C section. This must belong to the carboxylic part and the other side the alcohol part
This reaction only occurs when the mixture is heated to 50°C and in the presence of Sulfuric acid. However, not a large yield of ester is formed as an equilibrium is formed. This means in the reaction mixture both the alcohols, carboxylic acid and the ester is present. We can consider this reaction as a condensation reaction as a water molecule is produced
Hydrolysis of Esters
Esters can be hydrolysed either by using an acid or an alkali but, the products are slightly different depending on which method you use
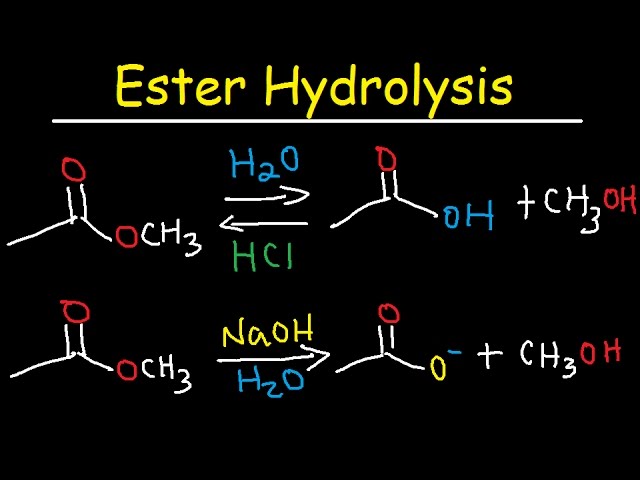
Acid Hydrolysis
When esters are hydrolysed using an acid like Concentrated sulfuric acid or HCl then a equilibrium is established. This is actually the same as the formation of esters because we use the same catalyst. So pure alcohol and carboxylic acids can not be obtained
Alkali Hydrolysis
The reaction does go to completion and so the esters are fully broken down to alcohols and carboxylic acids but the carboxylic acid can react with the alkali used. For example if we use NaOH, then it will react with the carboxylic acid to form sodium ethanoate
C2H5O2CCH3 + NaOH → C2H5OH + CH3COONa
Remember this reaction goes to completion and a sodium salt and alcohol is produced
Carboxylic acids
It is a weak organic acid that has the functional group -COOH
We don't need to know much about carboxylic acids because they act as typical normal acids but it is a weak acid and forms usually an equilibrium
It can react with metals and carbonates to form the salts:
Na + CH3COOH → CH3COONa + H2
It also can react with alcohols to form esters and more but now we will see some very important things such as the formation of carboxylic acids
Hydrolysis and Oxidation of Nitriles
We know that primary alcohols can be oxidised to form the carboxylic acids but it can also be formed from nitriles. For example, propanenitrile can be oxidised and hydrolysed in HCl (Hydrochloric acid) to form propanoic acid
CH3CH2CN + HCl → CH3COOH + NH4Cl
You don't need to know more than this
Reduction of Nitriles
This is a bonus part and not related to carboxylic acid but very helpful in the exam. The nitriles can be reduced to form amines
CH3CH2CN + [H] → CH3CH2CNH2
Simply what happens is the triple bond in the nitrile breaks and forms new single bonds with hydrogen. This almost like an addition reaction
Reduction of carboxylic acids
When primary alcohols are oxidised to produce carboxylic acids the reverse can occur also
When we add lithium aluminium hydride or LiAlH4 and dry ether, the carboxylic acids are reduced to the corresponding alcohols
CH3COOH + [H] → CH3CH2OH + H2O
The reason why we use dry ether is because LiAlH4 is a powerful reducing agent and so it is very reactive in water and causes it to explode. So to prevent it we use dry ether. A substitute for LiAlH4 is NaBH4 sodium boron hydride but the reaction is slower
