Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsEnthalpy Change
The energy change between the surrounding and the system at a constant pressure
During a chemical reaction, energy can be released or absorbed. So the energy of the system may decrease or increase. When a chemical reaction releases heat, we call it an exothermic reaction but, when it absorbs heat we call it an endothermic reaction
How we calculate enthalpy change is directly related to how we understand enthalpy of a system. Enthalpy of a sysytem is the energy in which the reactants or materials posses. During the reaction, the energy levels of the reactant or system may change and so this is known as the enthalpy change
ΔH = Enthalpy of products - Enthalpy of reactants
Energy can be released or absorbed to the surrounding. The surrounding can be:
1. The Solvent
2. The beaker or things in the solvent
3. Air around the beaker
We will now see the two types of reactions in depth
Exothermic Reactions
A chemical reaction that releases heat and increases the temperature of the surrounding is called an exothermic reaction. There are many exothermic reactions such as combustion or displacement reactions.
CH4 + 2O2 → CO2 + 2H2O
This is the combustion of methane and this acts as a fuel which releases alot of energy. We can see how much energy is released using an energy diagram or an energy profile diagram
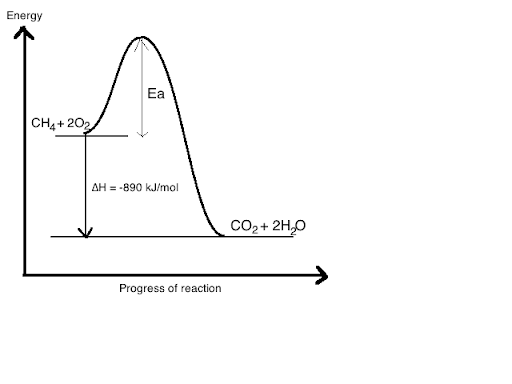
Another way to put this is that the energy needed to break the bonds is less than the energy released when forming bonds.
For any reaction to occur, they reactants must break their bonds and so this is endothermic as it requires energy. On the other hand, bond forming releases energy so it is exothermic
Endothermic Reactions
A chemical reaction that absorbs energy and reduces the surrounding temperature is an endothermic reaction. This means the system absorbs energy or heat and so the enthalpy must increase
In other words, the energy needed to break bonds is more than the energy released when forming bonds
A good example is ammonium salts dissolving in water:
NH4Cl(s) + H20 → NH4Cl(aq)
Standard Conditions
When we measure the enthalpy of a particular reaction this is usually done under standard conditions:
Temperature: 298K
Pressure: 1 atm
Concentration: 1moldm-3
For different types of reaction we define the enthalpy change differently but the general enthalpy change of a reaction is known as the " standard enthalpy change of a reaction ".
Standard Enthalpy Change of Reaction ΔH°r
The enthalpy change or energy change of a reaction in which the amount of reactants in the equation react to produce the reactants under standard conditions. The reactants and products are at standard conditions
For example let us take:
2H2 + O2 → 2H2O ΔHr= X
4H2 + 2O2 → 4H2O ΔHr = 2X
So the enthalpy change depends on your equation and it checks the energy released. It is a good idea to forget about the per mole part as it makes it more confusing. But how do we measure these enthalpy changes, we will see that next
Calculating Enthalpy Change
When we find the energy released from a chemical reaction we measure the temperature change of a known mass of water. We assume that all of the energy released from a reaction is absorbed by the water
E = mcΔT
We can either use mass in terms of Kg or grams but make sure you use the correct specific heat capacity value
An increase in temperature will be an release in heat and it will be negative but, a decrease in temperature will be a positive value so the equation is actually
E = -mcΔT
Let us see an example:
The mass of water is 2.5kg and the increase in temperature is 10K. What is the energy lost from the system
We will use:
E = -mcΔT
E = -2.5*4200*10
So this is -105000J of energy, meaning energy is lost from the system. If it was a drop in temperature then it would be 105000J so this is an increase in energy of the system. Seems confusing but this is basically the law of conservation of energy
There is another type of enthalpy change and this is for combustion reactions
Standard Enthalpy Change of Combustion ΔH°c
The enthalpy change when one mole of a substance reacts completely in excess oxygen under standard condition. The reactants and products are at standard conditons
H2 + ½O2 → H2O ΔHc= 4.2kJmol-1
These enthalpy change values are not the correct ones but are just examples
H2 + ½O2 → H2O ΔHc= 4.2kJmol-1
We need to make sure the equation has only one mole for the substance or fuel. This is a must and so the standard enthalpy change of combustion is only for one mole of combusted fuel
Always remember that we carry this out under standard conditions. We set up the experiment like the below
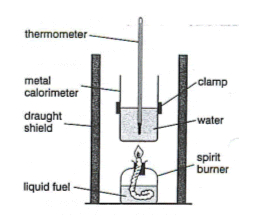
These are the steps used to carry out the experiment:
The spirit burner must have the cap on to prevent it drying out
Measure the initial mass of the spirit burner and record it
Add 100cm3 of water to a beaker. This must have an mass of 100g
Make sure you use a thermometer that is precise 0.01
Open the cap of the spirit burner and adjust the wick before it switched on. Lit the spirit burner
Keep it on until there is a 10°C increase in temperature and then close the cap of the spirit burner and measure the mass
Using this information we can calculate the enthalpy change
E = -mcΔT
E = -0.1*4200*10
So 4200J of energy is released and this the same as 4.2kJ
To find the energy per mole of substance of fuel reacted we need to know the molar mass of the fuel in the spirit burner and find the mass of the fuel reacted (Intial - Final mass of spirit burner)
Then we can find the enthalpy change:
ΔHC = 4.2/moles of fuel
This will give your answer in kJmol-1. As we can see, for this it is a must to divide it by the number of moles as the definition clearly defines for one mole. Remember this is not the same as ΔHr
Standard Enthalpy Change of Neutralisation ΔH°neu
The enthalpy change when two solutions, an acid and an alkali mix to produce one mole of water under standard conditions. The products and reactants are at standard conditions
When you mix an acid and an alkali, energy is released. So we use this to calculate the energy from neutralisation. Before we get in to detail, there are some assumptions:
1. The density of the mixture or solution is the same as water
2. The specific heat capacity is the same as water
By using these assumptions we can calculate the enthalpy change if we know the volumes of the two solution and the temperature change
When 25cm3 of NaOH is used to neutralise 25cm3 of HCl, there was an increase of temperature of 20°C
Sometimes the concentrations can be different and certainly in practice we do change them but, they will usually state the concentrations
So how do we find the mass, this is found by finding the total volume of the two solutions mixed which is 50cm3. So the mass is 50g. It is a good idea to use 4.18 instead of in kg
E = -mcΔT
E = -50*4.18*20
This releases 4180J of energy or 4.18kJ of energy. Then to find the enthalpy change, we need to divide by the number of moles of water formed. Most of the time it will be the same as the moles of the alkali or acid but, make sure you find the number of moles of water which is:
NaOH : H2O 1 : 1 0.05 : 0.05
So after finding it then divide it
ΔHneutralisation = 4.18/0.05
The answer is 83.6kJmol-1
Also note that this is for only one mole of water so even the balanced equation must be given for one mole of water only
More Standard Enthalpies
There are more standard enthalpy changes but you don't need to know them now. This will be very important in A Levels but currently we will just define each:
The enthalpy change when one mole of gaseous atoms are formed from their elements under standard conditions
½Cl2(g) → Cl(g)
This is actually half the bond enthalpy of Chlorine gas. This is important especially in A levels when they ask to calculate lattice energies and dissociations
Also this could simply be the energy to convert a solid to a gas. Also important in Lattice energies as they are used to calculate the ionisation energies
Mg(s) → Mg(g)
Enthalpy change when one mole of aqueous ions are formed from their gaseous ions under standard conditions
This is also important in A Levels as it is used to predict solubility
½Cl2(g) → Cl(aq)
The enthalpy change when one mole of a compound is dissolved to form an infinitely dilluted solution under standard conditions
NaCl(s) + H2O(l) → NaCl(aq)
This is somewhat important in Hess's law and is used to predict solubilty also
Some pointers
It is a great idea to know that these standard enthalpy change has a fixed or an exact balanced version of each equation except for standard enthalpy change of reaction. Another point to remember is that water is a liquid at standard conditions even if it is a combustion reaction. We did miss one important standard enthalpy change and that is Standard enthalpy change of formation which we will discuss in the next chapter
