Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsGiant Covalent & Ionic Structures
There are some giant covalent & ionic structures you need to know
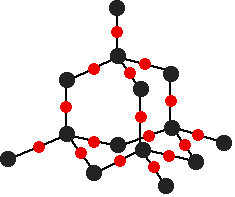
This is the structure of SiO2. This has a giant ionic structure
When you look at the structure we see that the silicon bonds to 4 oxygen atoms but why do we say SiO2?
In most questions, they might ask why we get this, so the reason is:
The Silicon atom is bonded to 4 Oxygen atoms and each Oxygen is bonded to 2 other silicon atoms. This leads to a ratio of 1:2
SiO2 has a tetrahedral structure and it is extremely strong but brittle. It is present in sand as quartz and is mainly used in Ceramic production as it is extremely strong and in the extraction of iron ( to remove carbon and other impurities and form slag )
This is an allotrope of carbon ( a different form of carbon atoms ). It has a similar strucutre to SiO2. This is the structure of Diamond:

Each carbon atom is bonded to 4 other carbon atoms covalently. In fact, the whole structure is linked with covalent bonds. For this reason, it has a tetrahedral structure and it is extremely strong but, brittle. It has a very high melting point as the whole structure is made out of covalent bonds so alot of energy is needed to break a large number of covalent bonds
Diamond is mainly used in drilling heads and also for cutting tools
It is an allotrope of carbon. The structure is:
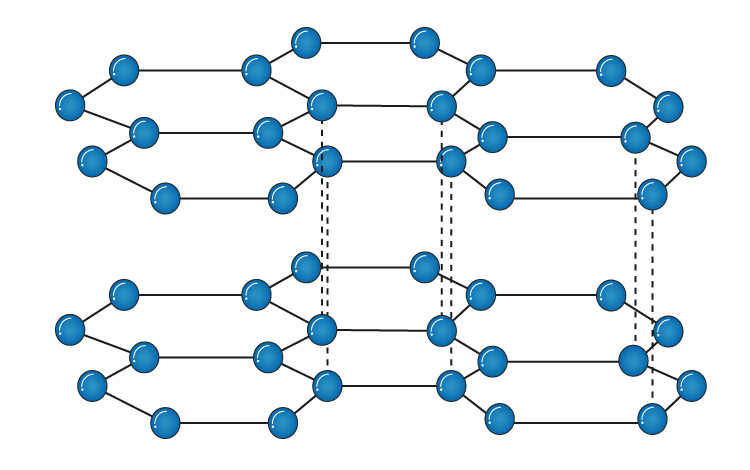
It is made out of layers held by weak vander waal forces. So the layers can easily slide off. That is why graphite is used as a lubricant as it is very slippery.
However, with in the layers, carbon atoms are bonded to 3 other carbons to form a hexagonal strucutre 2D layer. Due to this, the carbon has a delocalised electron that can carry charge and conduct electricity. Graphite is brittle as little energy is required to break the weak induce dipole forces but, it still has a very high meliting point as a lot of energy is needed to break the large number of covalent bonds
Graphite is used in pencil lead and as lubricants as it is slippery. It is also used as electrodes / slip rings as it conducts electricity
The only difference between Buckminster fullerene and fullerene is their structures:
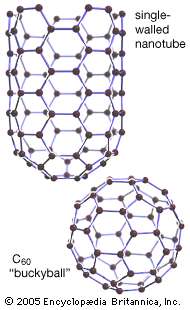
In buckminster fullerene or bucky ball, the carbon atoms are bonded to 3 other atoms and there molecules form a ball structure.
Bucky balls have weak induced dipole between the molecules so it has a low melting and boiling point and it can conduct electricity but very poorly as the molecules are seperated. Another point to remember is that a bucky ball can have 60-70 carbon atoms
Fullerene tubes also called nanotubes are somewhat strong as the long chain tubes contain covalently linked carbon atoms. They are extremely strong and have higher melting points. Many tubes can be used to make strong fibres and even strong clothing
Fullerene tubes are more reactive than graphite
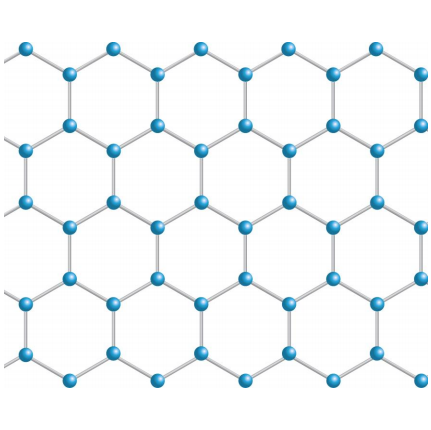
Also an allotrope of carbon. This is a single layer of the graphite structure
It is extremely strong for it's weight and has a very high melting and boiling point as the whole structure is covalently linked. This is like a 2D sheet structure and so it is very flexible. Lastly, graphene is very reactive even at low temperatures but can withstand high temperatures
Solid, Liquids & Gases
This is an extremely easy topic and you probably can refer any source for this. You need to know the properties of all the states and also you need to know how the atoms move inside. We will not dicuss more of this. If you want to know more, you can refer the physics A2 Section for the complete guide
