Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsKetones & Aldehydes
Ketones and aldehydes are very special type of organic compounds in chemistry. They are used as sugars or they are used for food essence and many other important things
Ketones and aldehyde have the C=O group in it but this is not the functional group. The functional group is -C-CO-C- and the -CHO functional group respectively. But it does react similarly in some reactions.
2,4 - DNPH
This can be used to test whether the sample contains either ketones or aldehydes. Most textbooks will say that this is used to test for the C=O group but this can be misleading as carboxylic acids and esters also have the C=O group and they do not take react in this reaction
When we add 2,4-Dinitrophenylhydrazine and if a ketone or aldehyde is present then a condensation reaction will occur. You do not need to know the mechanism but you need to know that this reaction releases water so it is a condensation reaction.
If a ketone or an aldehyde is present the color change from red to an orange ppt. If the sample does not contain any ketones or aldehydes the color remains red
There is a series of test that can be used to distinguish between aldehydes and ketones. we will see them each and try to remember these!
We use Copper sulfate (and also little bit of potassium hydroxide) known as the Fehling's reagent and add it to the sample
If an aldehyde is present, the color will change from blue to brown orange. This is because, initially the solution contains Cu2+ which is blue in color. When the aldehydes oxidise to carboxylic acids then the Cu2+ reduces to Cu+ which is brown in color
In fact, this is a redox reaction and you need to carry the experiment out in a warm/heated tube
If a ketone is present only, then there will be no color change. This is because the ketones can not oxidise to any other products so the color remains blue. This is a very important experiment used in biology to test for reducing sugars
We add the sample to aqueous silver nitrate. If an aldehyde is present then a silver lining or covering on the test tube will be formed. Initially the solution is colorless
But if it is a ketone there will be no visible change. Again the same principle is applied. The Ag+ ions are reduced to Ag to form a silver mirror while the aldehyde oxidises. This is a redox reaction and also must be warmed
This test is used rarely but we will still discuss it. This is used to oxidise primary and secondary alcohols. The same principle can be applied here. Acidified K2Cr2O7 will oxidise aldehydes but not ketones so the color changes from orange to green as Acidified K2Cr2O7 is reduced
The below table will gives a summarised note on the color changes
| Test | Intial Color | Final Color |
|---|---|---|
| 2,4 - DNPH | Red | Orange |
| Fehling's Test | Blue | Brown/Orange |
| Tollen's Test | Colorless | Silver mirror |
| Acidified K2Cr2O7 | Yellow/Orange | Green |
Nucleophillic additions
Luckily, you only need to know one example of this type of reaction. Ketones and aldehydes can only undergo nucleophillic additions. This is because it has a double bond and also the carbon atom in the C=O group is partially positive charged as oxygen is more electronegative than carbon. This makes it more likely to be attacked by nucleophiles
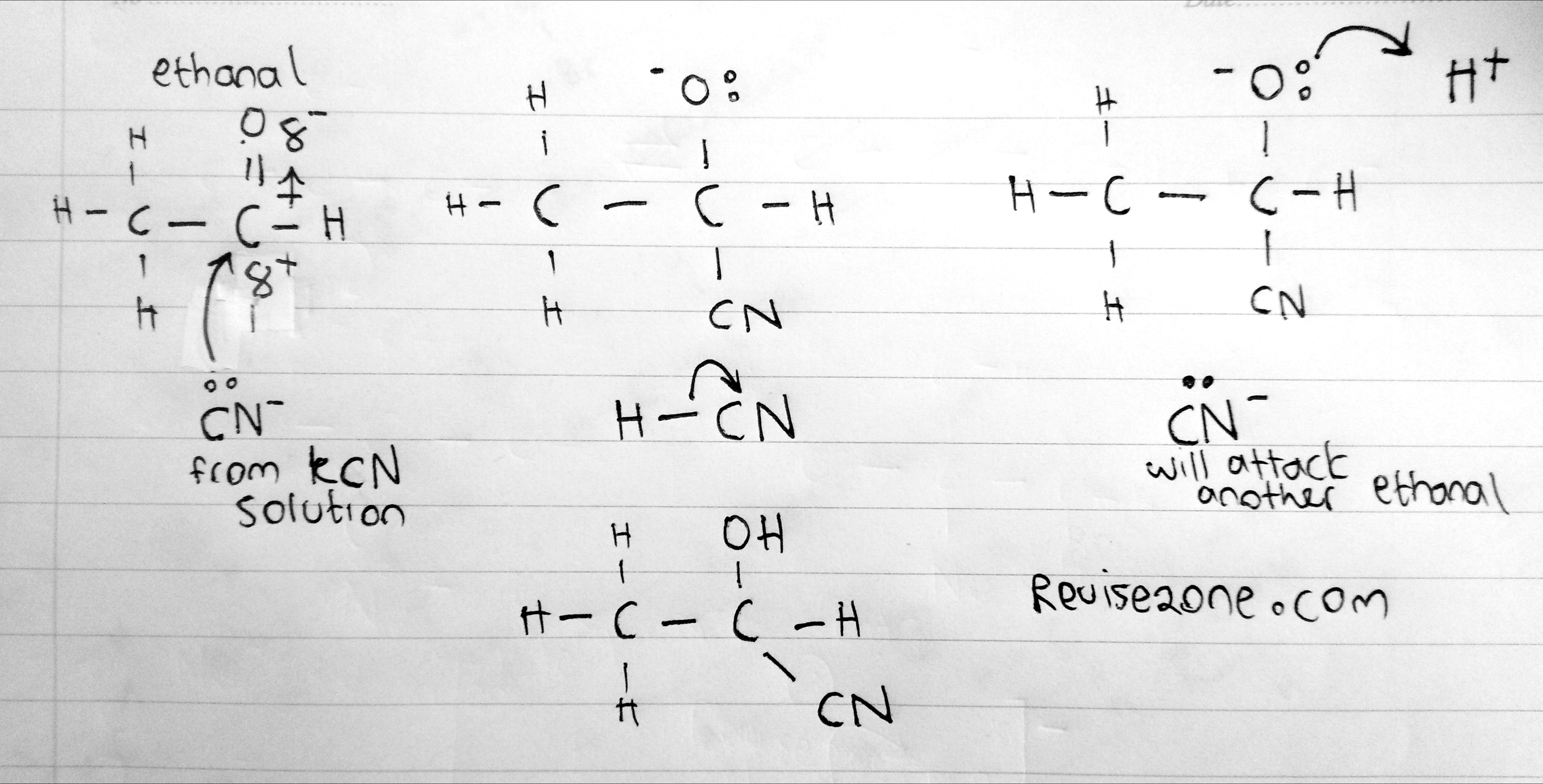
We will only see one example of nucleophile attacking and it is the Cyanide ion (CN-)
Reaction with HCN & KCN
When we add a ketone or an aldehyde to a solution of HCN and KCN then we will get a hydroxy nitrile. For example:
CH3CHO + HCN → CH3CH(OH)CN
So the Carbon double bond breaks and so the carbon attaches to the CN group. This will extend the carbon chain and have more carbon atoms. Also a hydrogen atom will bond to the Oxygen atom to form an OH group. This is why we call it a hydroxy!
The naming is quite is easy and it's good to know. First, remember that CN group has the highest priority and so it will have the lowest number but this is not that important as the OH group is also attached to the same carbon group. Due to the CN, the carbon number increases so we call it 1,1-hydroxypropanenitrile
Mechanism
You need to know how the mechanism works so here is the steps for this reaction
Reaction with NaOH & I2
When you react a ketone or an aldehyde with NaOH. It is hydrolysed or broken down by NaOH under reflux. The iodine gas is passed to react with the broken up parts to form Tri-Iodomethane and another halogen
But for this reaction to occur, we must choose the right reactants. This reaction will only work on ketones or aldehydes that have the CH3 Methyl group attached to the C=O group. Note that this must be a methyl group only and not any other group or else this reaction will not occur
According to this conditions the only aldehyde that can react is ethanal and the only ketones that can react are the secondary ketone which have -2-one part for example but-2-one. This is because only these products have exactly the methyl group attached to it
We will see the reaction of ethanal
CH3CHO + I2 → CHI3 + HCOONa
First the iodine gas is passed over ethanal to remove the hydrogen gas with iodine atoms this will result in CI3CHO. Then this CI3 will break due to hydrolysis by NaOH and so this will produce CHI3
CHI3 is a yellow solid with an antiseptic smell. This is used as a test for the presence of methyl groups in carbonyl compounds
Another important point is that this can be used as a test for secondary alcohols which are -2-ols for example, Propan-2-ol. This is because due to NaOH and high temperature, the secondary alcohols can oxidise to form ketones which has the methyl group. But rememeber this only works for alcohols that have the OH group attached to the 2nd carbon atom