Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsPeriodicity Notes
Across any period in a periodic table, there is an recurring pattern or trend for each property. We will discuss the main trends and patterns you need to know. We will use period 3 as our main example because this is what most exams follow
Ionisation Energies across the period
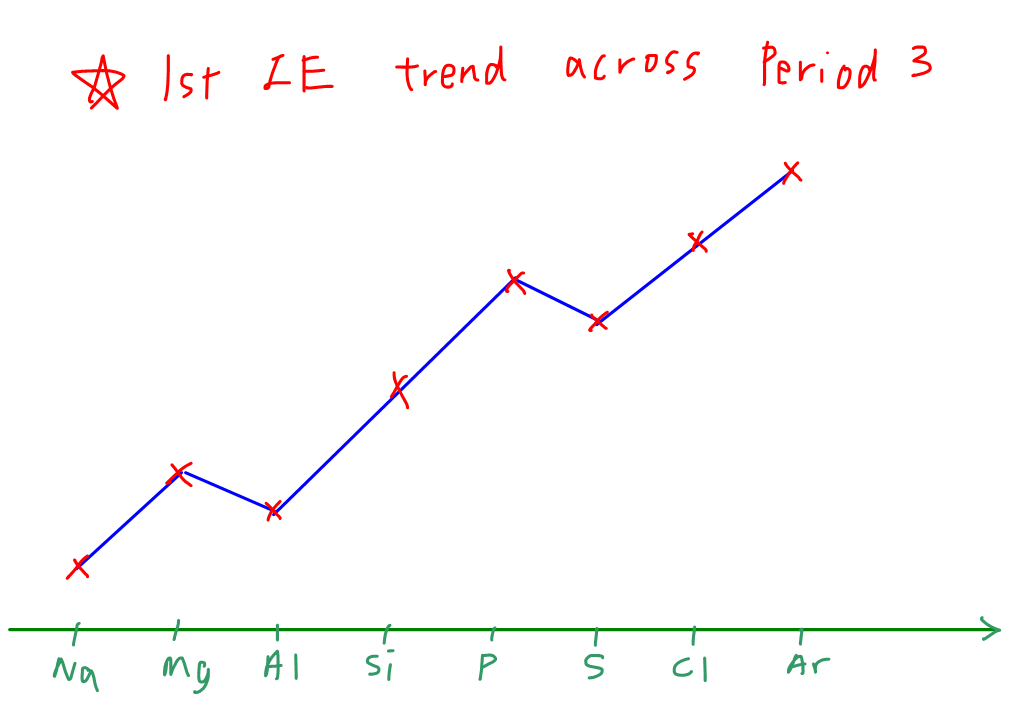
Across the period, the Ionisation energies generally increases. This is because, the shielding effect is more or less the same as electrons are added to the same principle Quantum shell and the atomic radius slightly decreases. Also the nucleur charge or the proton number increases causing a greater nucleur charge and attraction forces between the nucleus and the valence electrons. So more energy is required
The above explanation is enough to score marks. However, this is the general trend and so there are some exception you need to know
Exceptions to the rule
The exception are only for group 2,3 and group 5,6 elements
As you guessed it, group 3 elements has a slightly lower ionisation energies than the group 2 elements. Why? We will see the answer below
The electrons of group 3 elements is added to the P subshell instead of the S orbital. So due to slight increase in atomic radius and shielding effect being more significant than the increase in nucleur charge. The effective nucleur charge decreases and so lower attractive force
If you are answering a question, ensure that you mention the principal quantum shells of the Subshells you are talking about. For example, 3P and 3S. Also remember to use the word "significant" in your answer as it is very important
Now we will go to the group 5, 6 exception
The group 6 has a lower ionisation energy compared to group 5 elements. The reason is a bit different and it includes the 4th factor
For group 6 elements, the electron is paired with another electron whereas for group 5 elements the p subshell is half filled with individual electrons. So due to the spin-pair repulsion of the electrons, it reduces the effective nucleur charge or attraction forces and so less energy is required
This idea is very important, that is why remember that group 5 elements have a half filled P subshell
This pattern works for almost across any period. So that's why it is part of the periodicity notes!
Trends down the group
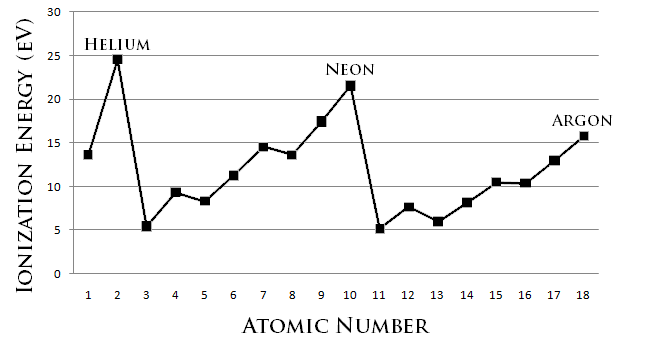
To simply put it the ionisation energies decrease down the group. Why? We will see the answer
The increase in atomic radius and the increase in shielding effect due to increase in inner core electrons is more significant than the increase in nucleur charge or proton number. So the effective nucleur charge and the attraction forces between the electrons and the nucleus decreases and so the ionisation energy decreases
It is that simple and that's all you need to know! Make sure you use the word significant!
Electronegativity Trends
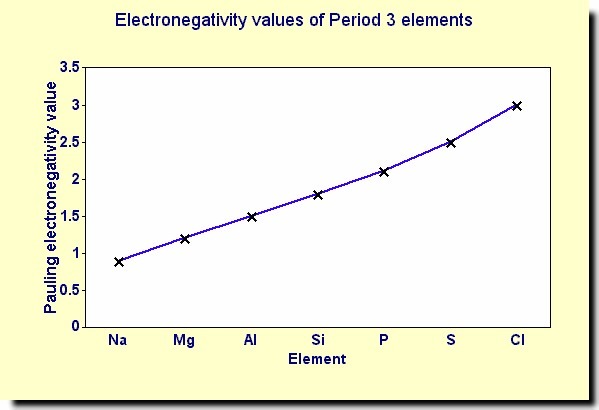

There is a clear increase in electronegativity across the period. Note that this does not follow the trend as the ionisation energy but the reasons are almost identical
Also NOF are the most electronegative elements
How is Atomic Radius determined
For different types of bonding the atomic radius is found differently:
Half of the distance between the centers of the two positive cations
Half of the distance between the centers of the two nuclei that is covalently bonded.
This will not give a precise radius as the orbitals are overlapping
Half of the distance between the centers of two atoms touching
Trends in Atomic Radius
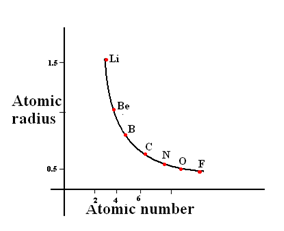
Atomic radius is a periodicity section but, we will still discuss it as it is related to ionisation energies
All you need to know that across a period, the atomic radius of elements decreases. Why? This is because, due to the increase in nucleur charge and shielding effect is more or less constant, there is greater attraction forces between the valence electrons and the nucleus and so greater pull. So the radius decreases
You will need to remember this graph and this is similar for any period
Exams question by asking to compare the atomic radius of 2 elements. For example, which one is larger in size, Na or Al. Even though it is tempting to think that because Al has more subshells, the atomic radius of Aluminium is less than Sodium. So Sodium is larger in size
Trends in Ionic Radius
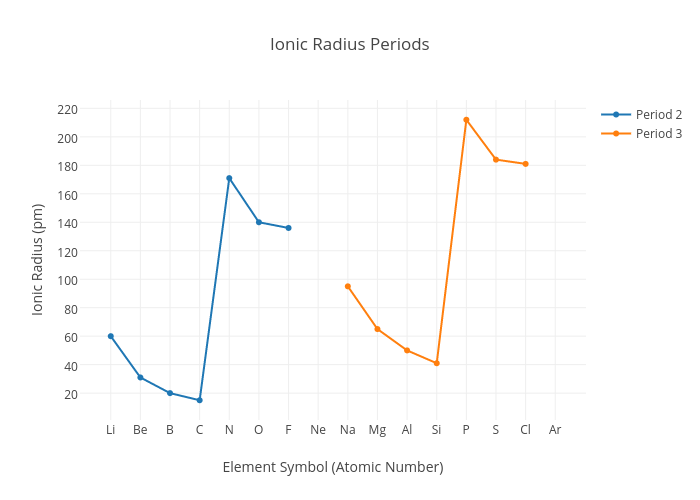
When comparing Ionic Radius, it is a bit different. Usually when elements form ions, they form the most stable electronic configuration
The Ionic radius of elements across a period also decreases but the anions have a larger radius than the cations. Remember the graph!
This is because, the anion has an extra principle quantum shell to hold more electrons and also there is greater shielding effect from inner core electrons
But for both Cations and Anions the Ionic radius decreases because the nucleur charge increases and the shielding effect is constant and so greater attraction and pull
Comparison between Ionic and Atomic Radius
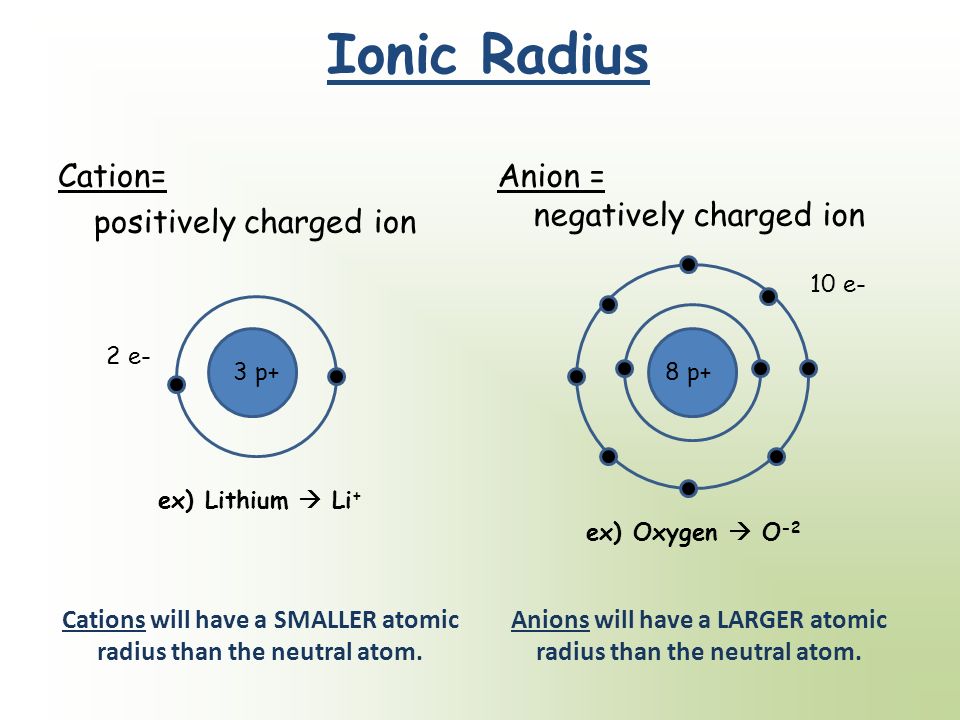
When we compare the Ionic or the atomic radius of a particular element, you need to consider whether it is a Anion or a cation!
Simply the cations has a smaller radius than the corresponding atom. Why? This is because, the cation has less electrons than the atom and so there is less repulsion force between electrons causing a greater EFFECTIVE nucleur charge on the valence electrons
The Anions has a larger radius than the corresponding atoms because, the anions have more electrons and so greater repulsion forces. This leads to a lower EFFECTIVE Nucleur charge on valence electrons so the radius is more larger
That's all you need to know. Please use the explanations above as they are from the markshemes!
Reactions with Oxygens
We will see how elements across the period react with oxygen. We will take period 3 as the example
Na - Burns with a yellow flame Mg - Burns with a bright white flame Al - Slowly oxidises Si - Slowly oxidises P - Burns with a yellow flame S - Burns with a blue flame
Solubility of oxides
We will now see how the solubility of oxides changes across a period
| Compound | Solubility |
|---|---|
| Na2O | Very Soluble |
| MgO | Slightly Soluble |
| Al2O3 | Insoluble |
| SiO2 | Insoluble |
| P2O5 | Soluble | SO2 | Soluble |
pH of Oxides
The pH of the oxide depends on whether the compound is ionic or covalent
| Compound | Nature |
|---|---|
| Na2O | Basic |
| MgO | Basic |
| Al2O3 | Ampthoreic |
| SiO2 | Acidic |
| P2O5 | Acidic | SO2 | Acidic |
You need to know that Aluminium oxide and Silicon dioxide does not dissolve but does react
When a metal oxide such as sodium dissolve in water, OH- ions are released.
O2- + H2O → 2OH-
That is why Na2O and MgO are basic...
Period 3 Chlorides
There is a clear trend in the acidity of chlorides in water across the period
| Compound | pH |
|---|---|
| NaCl | 7 |
| MgCl2 | 6 |
| Al2Cl6 | 4 |
| SiCl4 | 3 |
| PCl5 | 3 | SCl2 | 3 |
What you need to remember is that all Chlorides except the group 1 chlorides are acidic. Group 1 Chlorides are always neutral, the acidicity increases across the period and you need to know these pH values for each element
Another Point to remember is that, the chlorides are HYDROLYSED by water which is a reaction that makes the solution acidic. Only Sodium Chloride does not hydrolyse but just dissolves in water
Oxidation Number of Chlorides & Oxides
We need to remember that the more electronegative atoms are always negatively charged. We will see the Oxidation of each element in their chlorides:
| Compound | Oxidation Number |
|---|---|
| NaCl | +1 |
| MgCl2 | +2 |
| Al2Cl6 | +3 |
| SiCl4 | +4 |
| PCl5 | +5 | SCl6 | +6 |
We will see the oxidation number of Oxides. It is very similar
| Compound | Oxidation Number |
|---|---|
| Na2O | +1 |
| MgO | +2 |
| Al2O3 | +3 |
| SiO2 | +4 |
| P2O5 | +5 | S02 | +4 |
Trends in Bonding of Elements
The physical properties usually depend on the bonding involved in each group. Usually, there is a same type of bonding for each group
| Group | Bonding |
|---|---|
| 1 | Metallic |
| 2 | Metallic |
| 3 | Metallic |
| 4 | Giant Covalent |
| 5 | Simple molecular |
| 6 | Simple molecular |
| 7 | Simple molecular | 8 | Monoatomic |
By using this, we can predict the trend of the melting and electric conductivity across the period.
Melting and Boiling points
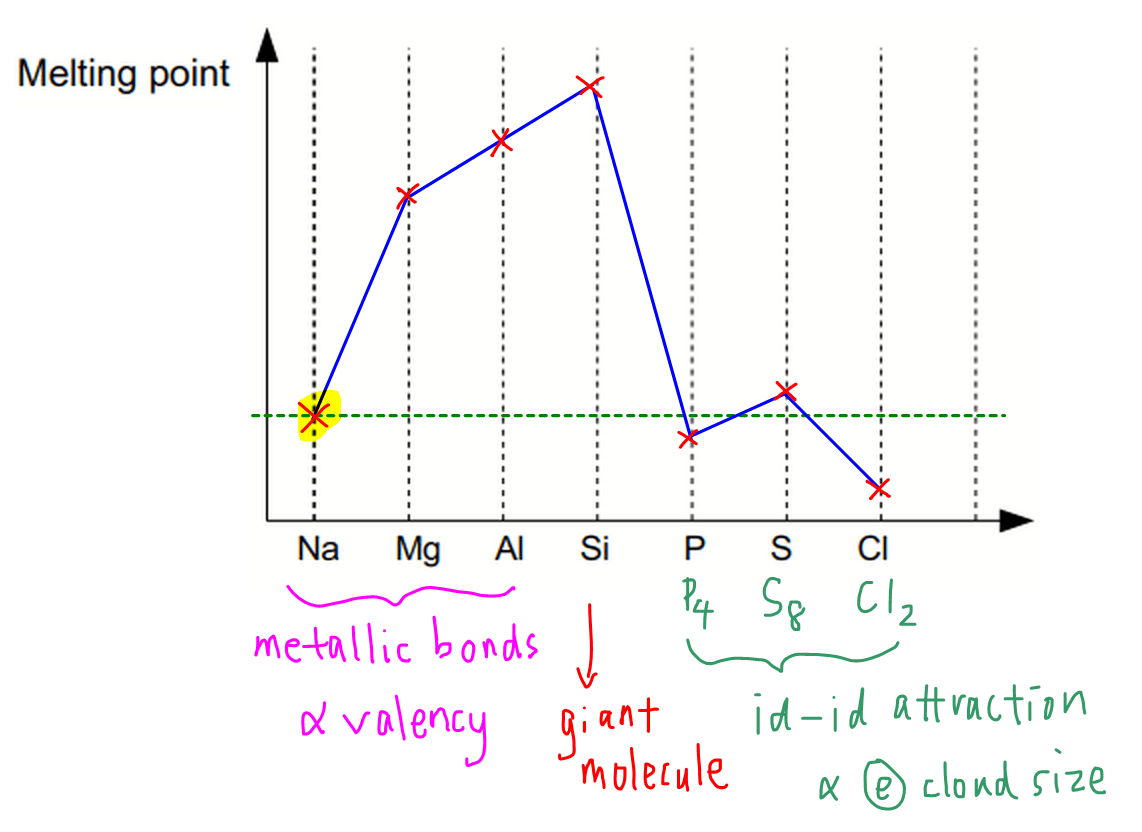
The melting point from Group 1 to Group 5 increases. We will see why
The Group 1 , 2 & 3 have metallic bonding but from group 1 to 3, the charge of the ions formed by the metals increases ( Na+, Mg2+, Al3+ ). So higher the charge, the higher the charge density and greater electrostatic forces between metal ions. This makes it much more stronger and have a higher melting point.
For group 4, as it is a giant covalent structure (full of covalent bonds), this makes the structure extremely strong and have the highest melting & boling point
For group after the Group 4, the melting point decreases as they are simple molecular strucures. However, the group 6 element has a slightly higher boiling and melting point than its usual trend.
Electric Conductivity
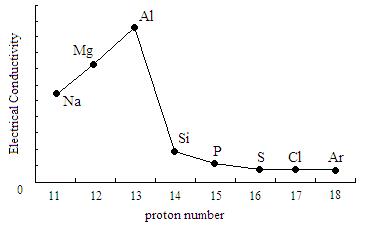
This also depends on the bonding. From group 1 to 3, the electric conductivity increases as the metal ions have a greater charge and so there is more free delocalised electrons to carry charge.
From group 3 onwards the electric conductivity decreases. This is because giant covalent and simple molecular structures do not have free mobile electrons to carry charge
