Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsChemical Bonding & Hybridisation
We need to know that there are 3 types on bonding:
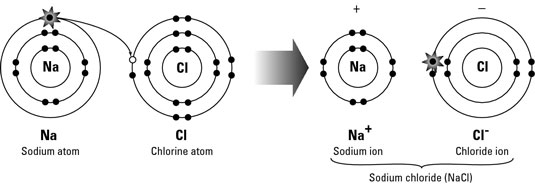
The electrostatic force attraction between the oppositely charged Cations and Anions
This is also called Electrovalent Bonding
Opposite charged ions attract and so they create ionic bonds between the ions
Usually Ionic bonding occurs between a metal cation and a non metal anion but, it can also occur for polyatomic cations which do not contain any metal ions. For example, the Ammonium ion is +1 charged
When we draw the ionic bonding we need to indicate that we use ions. So we use brackets. Also, we show the electrons gained by using different symbols . Eg - we use circles or crosses
We also indicate the sign or the charge of the ion
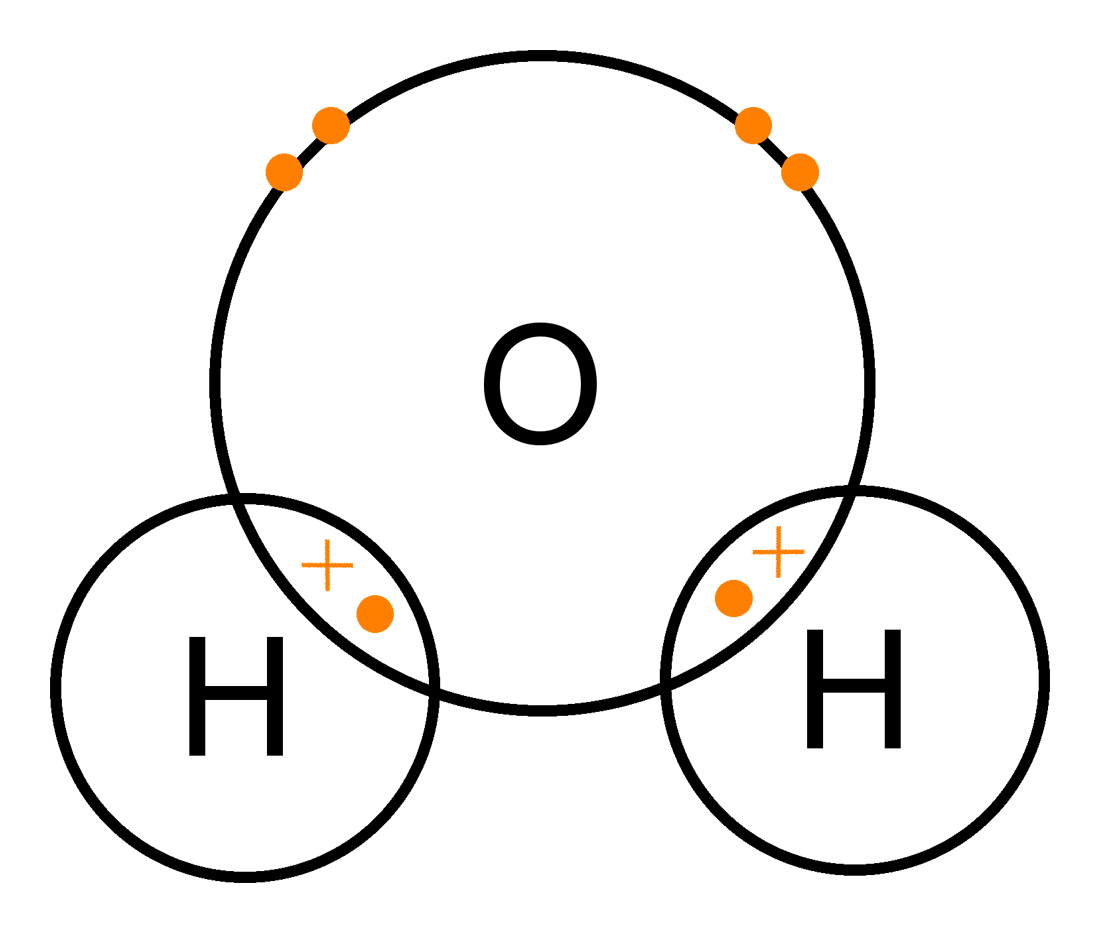
The Electrostatic force attraction between the shared pair of electrons and the two positive nuclei
When we represent Covalent bonding, we show the transfer of electrons using dots and crosses. The dots and crosses will be defined for each atom by the question
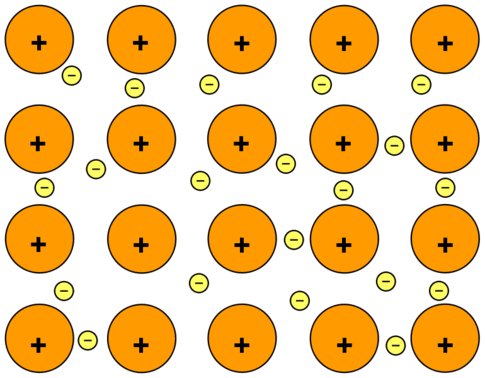
The electrostatic attraction forces between the sea of delocalised electrons and the lattice arranged positive cations
The cations are formed when the metal atoms lose their valence electrons and align in a lattice structure. The electrons form the sea of delocalised electrons which can carry electricity
Dative Bonding or Coordinate Bonding
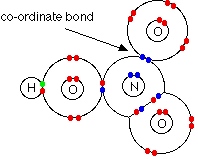
The electrostatic force attraction between the two species in which one of the two species donates the pair of electron that is being shared
Dative bonding is a specific category under covalent bonding. This is when one species donate both electron. This is usually done by an nucleophile which contains a lone pair
We indicate the dative bonding using an arrow. The Direction shows us the direction in which the lone pair is donated TO
We will see an example where dative bonding occurs
HO + H+ → H3O+
The water contains 2 lone pair which donates one of its lone pair to the positive hydrogen ion and forms a dative bond. We get a new ion called the hydroxonium ion!
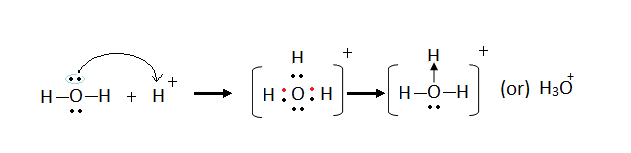
The hydroxonium ion has an overall charge of +1 because of the positive hydrogen ion. This ion is very important and is released when an acid dissolves in water
The next ion we want to look at is the dimer - Al2Cl6
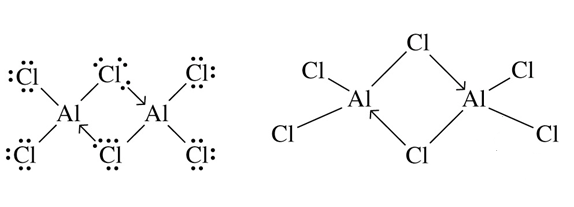
This is a bit complicating. The Chlorine atom donates a lone pair to the other Aluminium atom and forms a dative bond and the other chlorine atom forms another dative bond with the aluminium atom. So there are 2 dative bonds for each dimer molecule
Orbital Overlapping
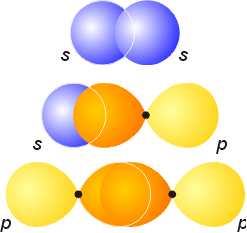
Whenever 2 S orbitals or an S and P orbital or P and P orbital overlap, they form a sigma bond
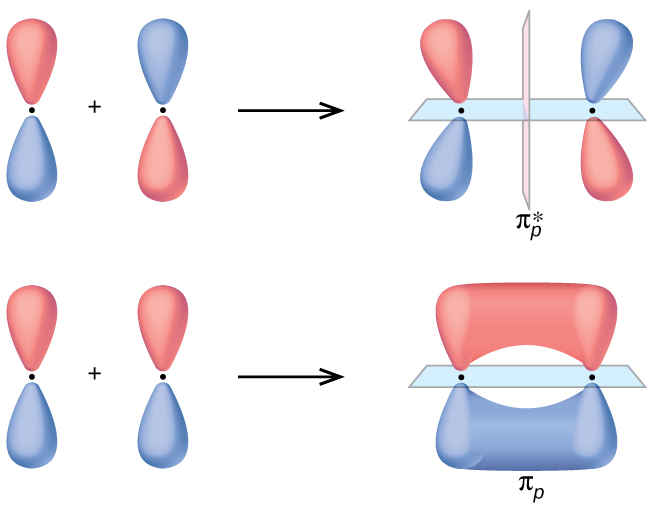
A π bond is only formed when the P orbital overlaps laterally with another P orbital
We need to know the shape of the orbitals overlapping. Make sure when you draw it, you highlight the overlapping of the two orbitals
Always keep in mind that the π bond is weaker than the sigma bond and its created by lateral overlapping of 2 P orbitals
Hybridisation
Hybridisation is not a real concept but a theoretical concept created to explain bonding in some compounds
Let us take the example methane. Methane has 4 hydrogen atoms bonded to the carbon atoms
When we look at the Electronic configuration of carbon we see that bonding can only occur when there is an unpaired electron in each orbital
So we may assume the electron from the S Subshell and the 2 electrons in P Subshell undergo bonding, but they won't make the same type and number of bonds
To explain this we have made a theory which explains why all of the bond in an methane molecule are the same using hybridisation
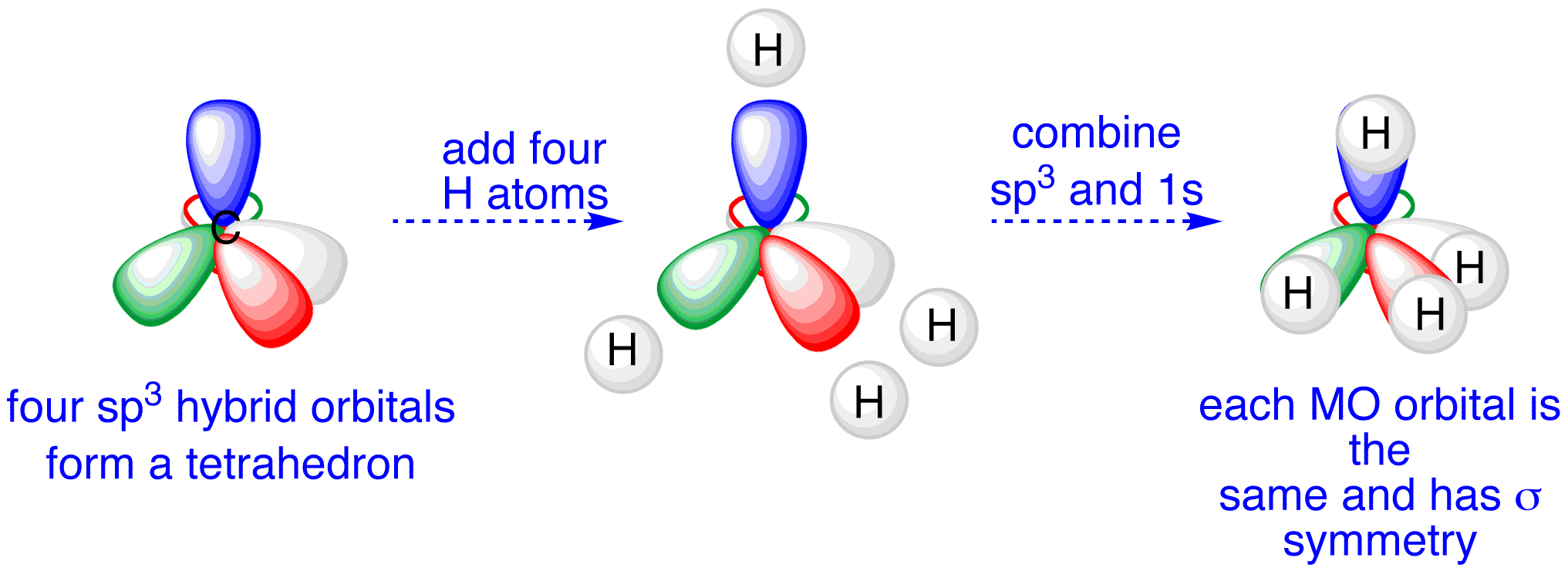
Below are some points you need to mention
1. The electron in the 2S orbital gets excited and moves to the empty P orbital known as the excited state
2. The three P orbitals and the S orbitals hybridise to form 4 new hybrid Orbitals. The Hybrid orbitals have energy between the P and S orbitals
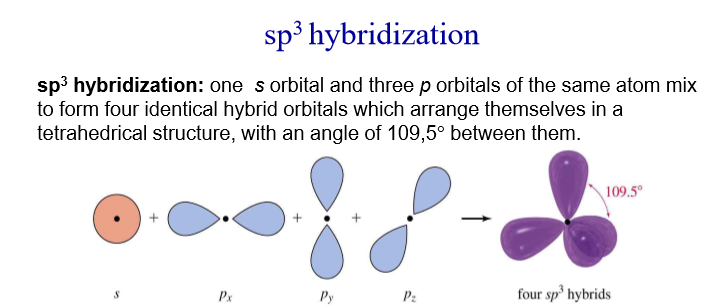
3. These 4 Hybrid orbitals are called Sp3 orbitals. We call them Sp3 because 3 P orbitals and 1 S orbital hyridised to form 4 of them
4. These 4 Sp3 orbitals then bond with Hydrogen atoms forming Sigma bonds
Sp2 & Sp Hybridisation
There are more compounds which don't fit the Sp3 hybridisation, for example ethene and alkynes
We will take Ethene to explain Sp2 Hybridisation
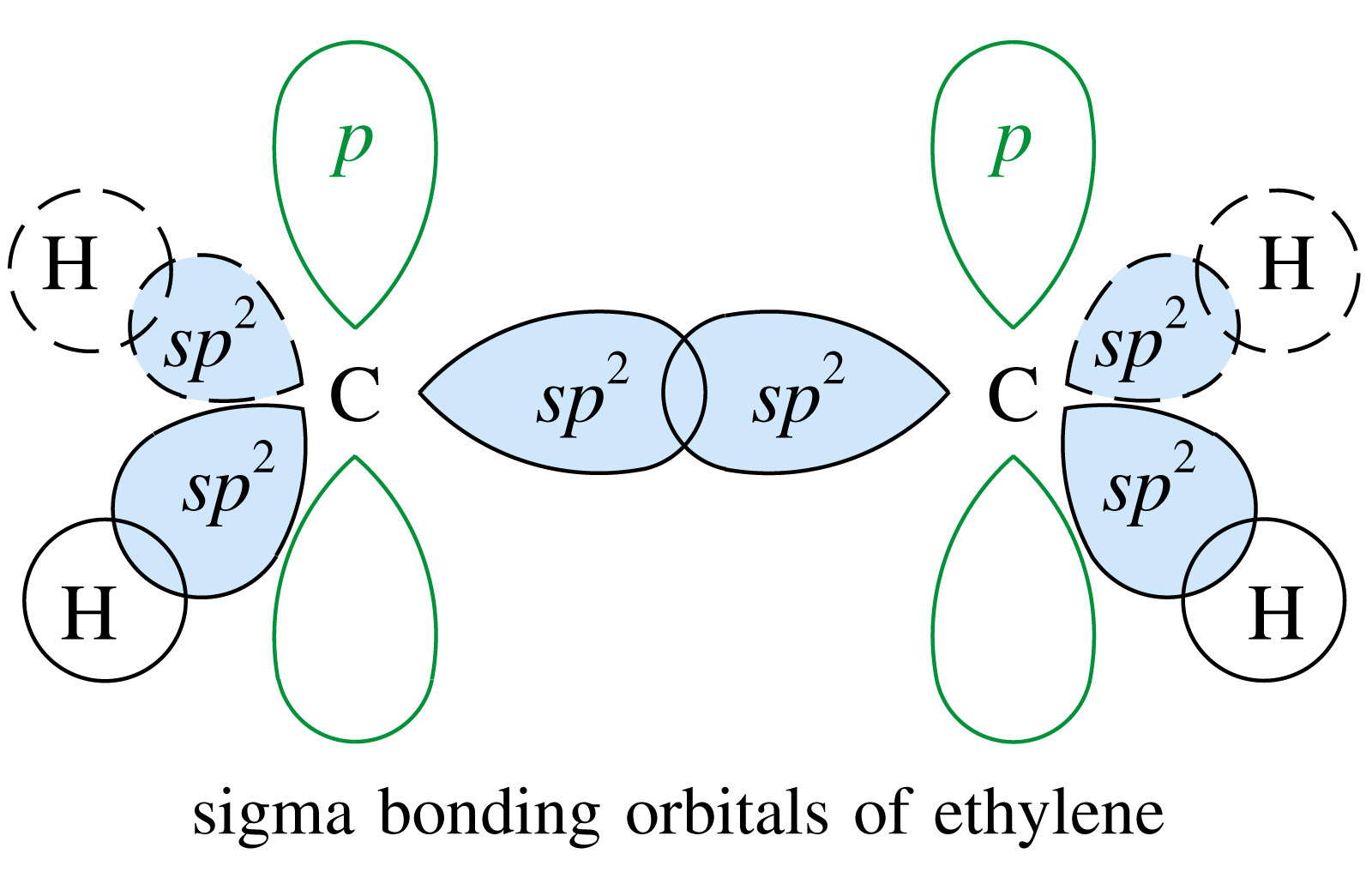
The first step is the same as Sp3 Hybridisation
1. The electron in the 2S orbital gets excited and moves to the empty P orbital known as the excited state
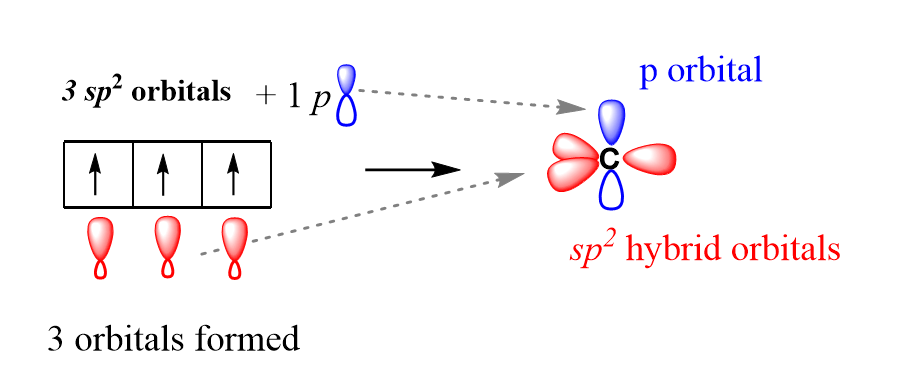
2. The Two P orbitals and the S orbitals hybridise to form 3 new hybrid Orbitals. The Hybrid orbitals have energy between the P and S orbitals
3. These 3 Hybrid orbitals are called Sp2 orbitals. We call them Sp2 because two P orbitals and 1 S orbital hyridised to form 3 of them
4. These 3 Sp2 orbitals then bond with Hydrogen atoms forming Sigma bonds
5. The remaining unpaired P orbital overlaps laterally with another unpaired P orbitals. This is why there must be two carbon atoms undergoing hybridisation
For any Double bond, there is always a π and a sigma bond present and it is always Sp2 Hybridisation
There are some few points you need to remember in Sp2 Hybridisation:
1. There is restriction in rotation around the bond. This results in Cis-trans isomerism
2. During reaction, always the π bond breaks, this is because due to the carbon double bond it has an high electron density, which acts as a nucleophile
3. We represent the π bonds using two electron clouds, one at the top and one at the bottom
You might have notice a pattern here and you might be able to crack the Sp Hybridisation
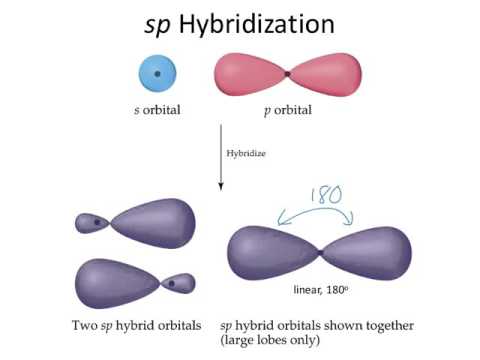
1. The electron in the 2S orbital gets excited and moves to the empty P orbital known as the excited state
2. One P orbitals and the S orbitals hybridise to form 2 new hybrid Orbitals. The Hybrid orbitals have energy between the P and S orbitals
3. These 2 Hybrid orbitals are called Sp2 orbitals. We call them Sp2 because 1 P orbital and 1 S orbital hyridised to form 2 of them
4. These 2 Sp orbitals then bond with Hydrogen atoms forming Sigma bonds
5. The remaining Two unpaired P orbitals overlaps laterally with another two unpaired P orbitals. This is why there must be two carbon atoms undergoing hybridisation
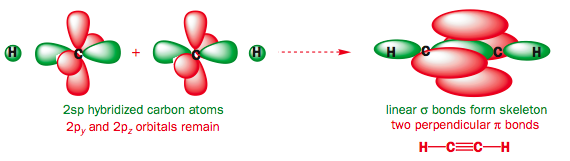
This will result in 2 π bonds and 1 sigma bond. Always when there is a triple bond it is Sp Hybridisation and a good example is an alkyne
Angles Between Bonds
You need to know the angles between the bonds in Sp3, Sp2 and Sp Hybridisation. This can be done by a bit of logic and understanding
For Sp3 Hybridisation, there are all together 4 Hybridised Bonds. These bonds are identical and so they repel each bond equally forming a tetrahedral shape with the bond angle 109.5°. A different idea could applied to Sp2 and Sp Hybridisation
For Sp2 Hybridisation, there are 3 Hybridisation bonds which space evenly to form a triagonal planar with the bond angle 120°. The P orbital is positioned perpendicular to the triagonal planar with the bond angle 90° but, questions usually ask the bond angle between the Sp2 Hybridised orbitals
For Sp Hybridisation, there are only 2 hybrid orbitals, so logic tells us that they are going to be arranged as far as possible which forms a linear shape of bond angle 180°. The 2 P orbitals are positioned perpendicular to the hybridised orbitals
We have predicted the bond angles and shapes of some molecules using basic common sense, but it is difficult to predict the shapes of complex molecules using logic so that is why we use the VSEPR Theory ( Valence shell electron pair repulsion theory ). Which we will see more below
VSEPR
There are two types of electron pairs:
- Bond pair
- Lone pair
These are electrons which partcipate in bonding with another atom. For example, the hydrogen atom bonds with the other hydrogen bond using a single bond pair (electron pair)
These are electron pairs that don't undergo bonding. This type of electron pair has the highest electron density and so this exerts the highest repulsion forces
The order of repulsion forces must be known as it helps us to predict the shape of the molecule. In fact the shape of the molecule depends on two things, the number of electron pairs around the central atom ONLY and the type of electron pair
Why do we care about the type of electron pair, this is because different types exerts different forces. Some are very repulsive and some are not
The more repulsive it is the futher the two electron pairs spread out to become stable. This idea of stability comes from advance physics, which will discussed under Columb's law in A2 Physics
This is the order of the repulsion between electron pairs (least repulsive first):
1. Bond - Bond Pair
2. Bond - Lone pair
3. Lone - Lone pair
This means the spaces between the lone pairs will be greater than the spaces between bond pairs or between bond - lone pairs!
We will give a list of the most important molecules shapes and their bond angle
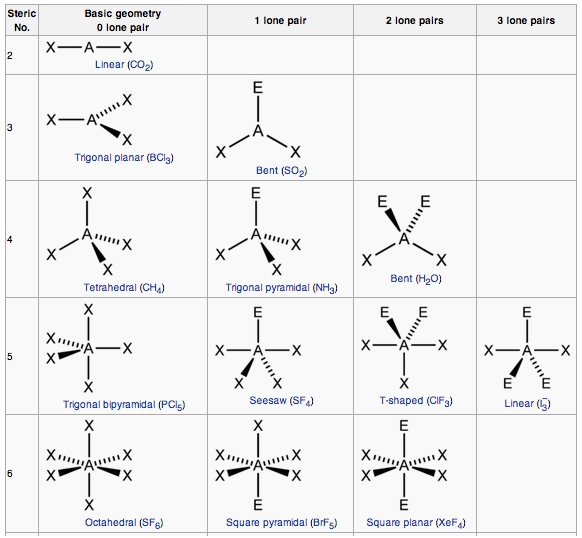
The reasoning can be applied to almost anything. First you need to see the number of lone and bond pairs around the CENTRAL atom of the molecule ( as that is what determines the shape ). Then You identify the type of electron pair and the ratio between bond and lone pairs. Using this knowledge you can predict its shape
Also, another point to remember is that the more lone pairs the central atom contains the smaller the bond angle. In fact there is a relationship you can remember
1. Tetrahedral (4:0) - 109.5°
2. Triagonal pyramidal (4:0) - 107°
3. Bent or V Shaped (4:0) - 104.5°
A very simple relationship can be established, the bond angle decreases by 2.5 for every lone pair
How to answer these Questions?
This question can be fully answered if you include these point in your answer. They will give an example of a molecule for us to comment on
State the bond angle and the Molecule shape
State the number of Bond Pairs and the number of bond pairs
State which electron pairs have the greatest repulsive forces between them and explain them more
State the lone pairs which have the greatest repulsion between them , so they spread out and take more space compared to other electron pairs to become stable
