Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsAlkanes
They are Saturated Hydrocarbons with the General Formula CnH2n+2
The reason why we say alkanes are saturated is because there are no carbon double bonds in the compound and so the maximum number of hydrogen atoms are bonded (SP3)
There are 2 types of organic compounds:
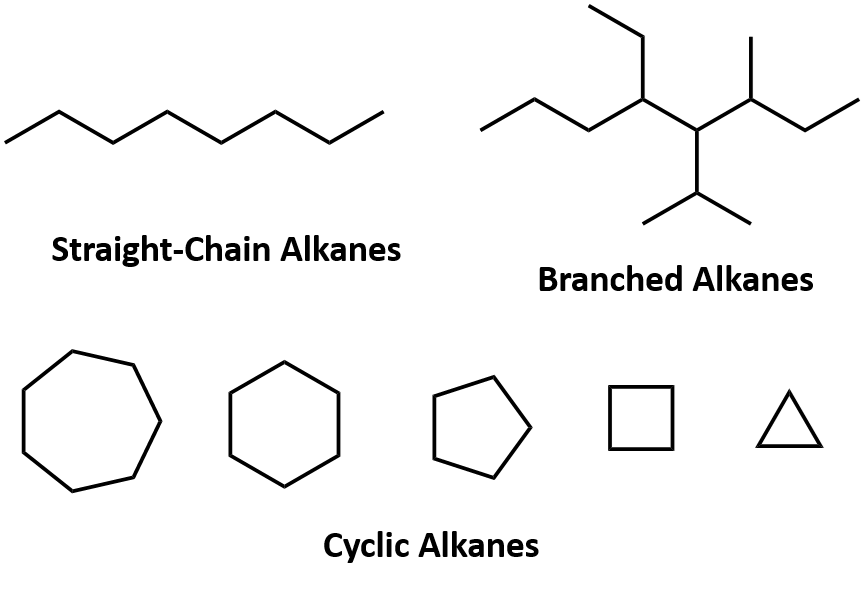
These are compounds that do not contain any benzene rings and are made out of carbon atoms branched in either a straight line or in an cycle
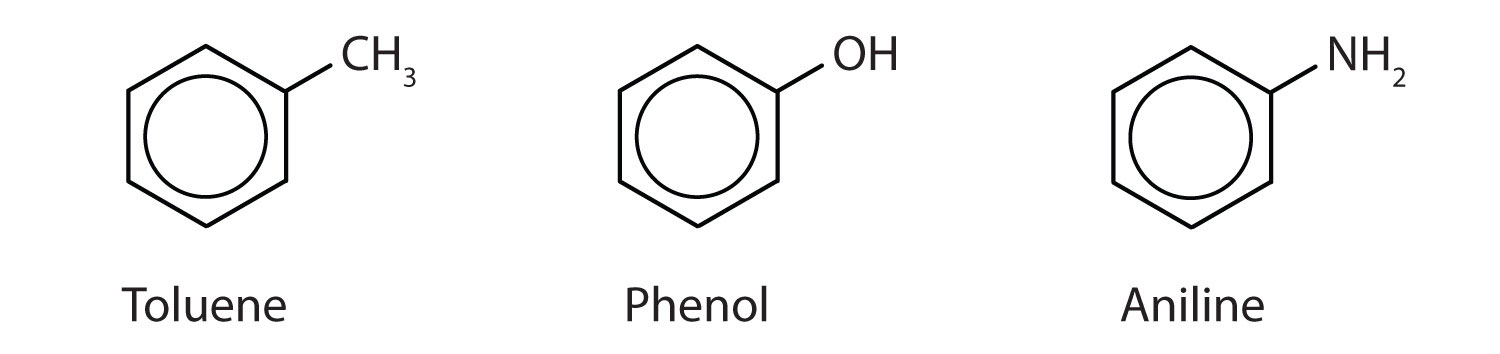
Compounds that contain the benzene ring
These are present in crude oil and can be broken down to futher sub-groups known as classes of hydrocarbons but, before that we need to know what is crude oil!
Crude oil / Petrolleum
A mixture of hydrocarbons
Crude oil can have these following types or classes of hydrocarbons:
1. Alkane
2. Alkene
3. Cycloalkane
4. Aromatic Compounds
When they say class of hydrocarbons then we need to choose from the above.
Crude oil can be seperated into many fractions depending on their boiling points. A fraction is part of crude oil that is distilled during the fractional distillation of crude oil
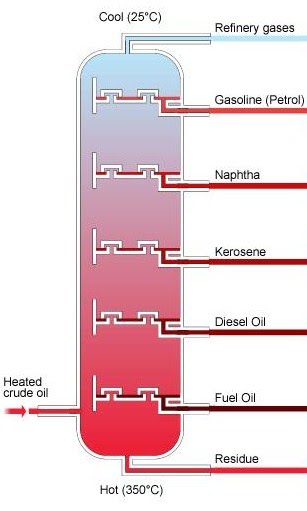
You need to remember this diagram and the order in which each fraction is obtained
Uses of Each Fraction
| Fraction | Uses |
|---|---|
| Natural Gas | Used as a fuel for domestic cooking |
| Gasoline | Used as a fuel in petrol engines |
| Naphtha | Used to make chemicals |
| Kerosene | Used as Jet fuel |
| Diesel | Used as a fuel in diesel engines |
| Fuel oil | Used as a fuel in ships and used as a lubricant and a polish oil |
| Bitumen | Used to make roads and roof tiles |
Some basic trends down the fractionating tower is that the boiling and the melting point of the fraction increases which means volatility decreases. Also the density increases as the carbon chain increases. The viscousity also increases.
A chemical property you can comment on is the flammability and flammability decreases down the group
Reactions of Alkane
Alkanes do not have any special functional groups so it is the least reactive hydrocarbon
In fact, there are only 3 type of reactions in which an alkane can undergo
There is incomplete and Complete combustion. You will need to know the products for each reaction so we will take methane as the example
When Methane react in excess oxygen (Complete Combustion):
CH4 + O2 → CO2 + 2H2O
When methane is burnt in limited oxygen, it has several different equations:
2CH4 + 3O2 → 2CO+ 4H2O
2CH4 + 2O2 → 2C+ 4H2O
The reason why I have given two equations is that sometimes the questions may ask the production of a black substance only which is carbon ( or soot )
But the general equation is the first one
When an Alkane is reacted with a halogen such as Chlorine in the presence of sunlight or UV light. Then a free radical substitution reaction occurs that results in several different mixtures of Halogenoalkanes
CH4 + Cl2 → CH3Cl + HCl
But this reaction can produce many more different products such as CH2Cl2, CHCl3 and CCl4. This is because due to UV light the Chlorine bond in the Cl2 breaks homolytically to form chlorine free radicals that can react and attack stable products
In this reaction there are 3 steps which we are going to talk about and they are:
1. Initiation
2. Propagation
3. Termination
Initiation Step
In this step, the Chlorine molecule breaks into free radicals due to the presence of UV light
Cl2 → 2Cl*
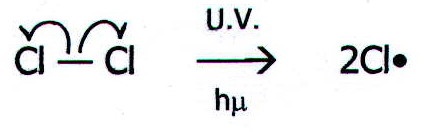
These Chlorine free radicals are highly unstable and highly reactive and easily attack other atoms
The bond breaks by homolytic fission meaning that each chlorine gets an electron from the bond. This stage is not considered to be a redox reaction
Propagation
The Chlorine free radicals attack other stable products such as methane to make more free radical ( the methyl free radical ) this causes a chain reaction in which the newly formed free radical can attack another substance and produce another free radical
CH4 + Cl* → *CH3 + HCl
*CH3 + Cl2 → CH3Cl + *Cl
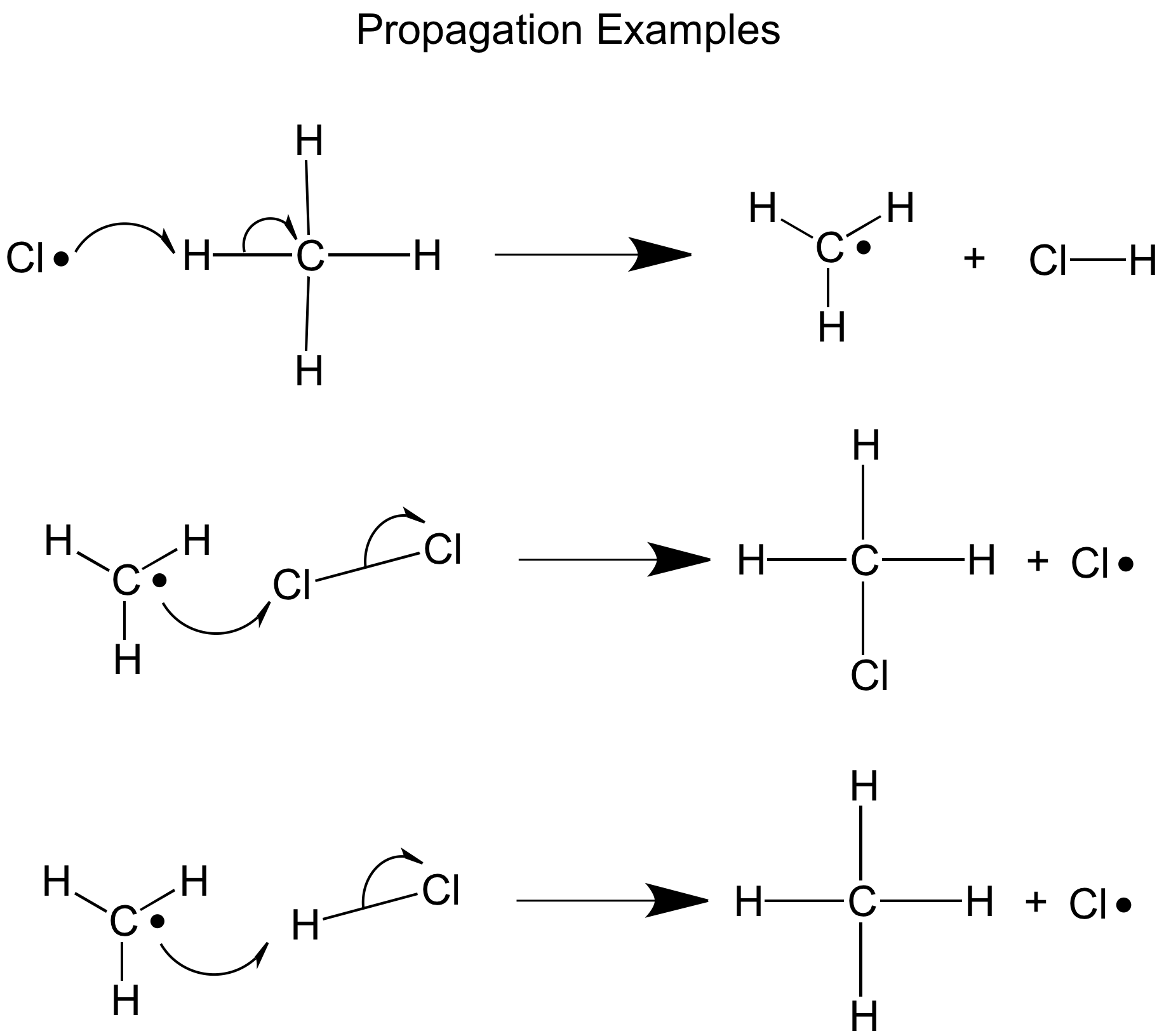
Remember this stage is a redox reaction as the chlorine does reduce
Termination Stage
When the concentration of free radicals is high and the concentration of stable products is low. Then the free radicals react with other free radicals to make a stable product and no more free radicals
*CH3 + *CH3 → CH3CH3
*CH3 + *Cl → CH3Cl
*CH2Cl + *Cl → CH2Cl2
There is a mixture of products that can be formed.
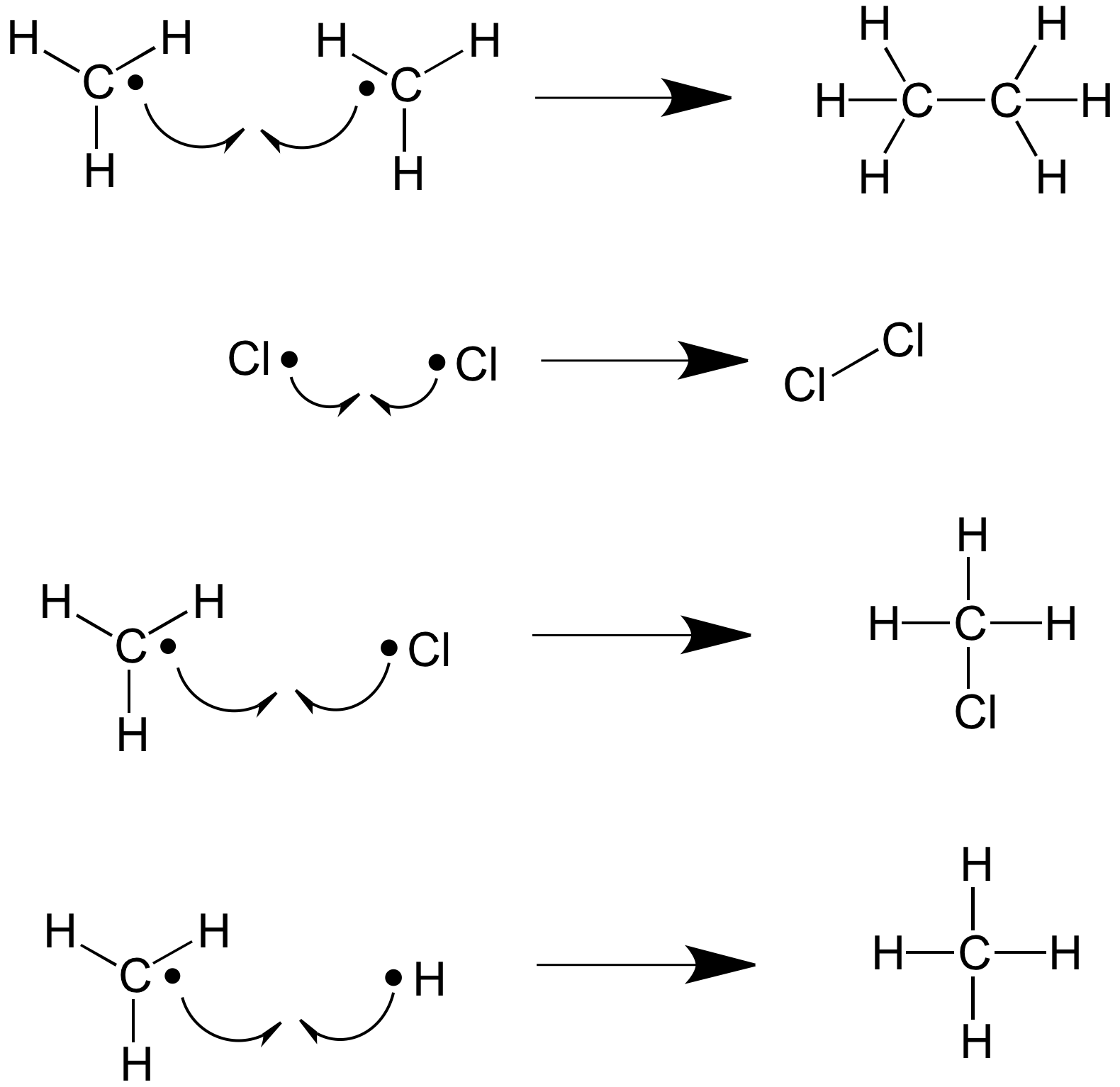
Mechanism
We need to know how to draw these proccess for each stage and this below diagram will go through it
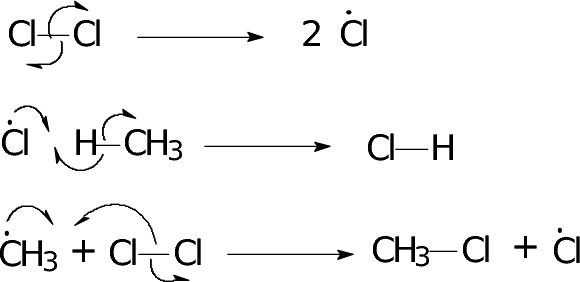
Some things you need to know is that a HALF Curly arrow is used to show it is Homolytic fission and the arrow must be from the center of the bond to the corresponding atom. This atom becomes a free radical. When a free radical reacts then the arrow must start from the free radical star to the atom it is going to bond with
Process of breaking a large carbon chain to smaller useful carbon chains where one of them is always an alkene
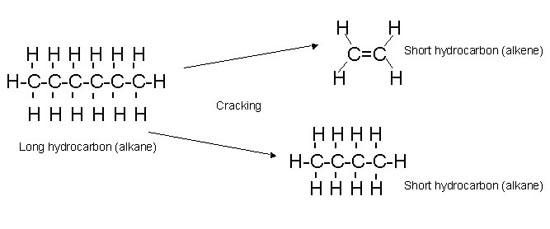
Large carbon chains such as bitumen are useful but less in demand compared to lighter fuel and gasoline. So we use cracking to break them into smaller useful ones
C20H42 → C5H12 + C4H8 +C11H22
As we can see the carbon atoms and hydrogen atoms must be conserved and always an alkene MUST be produced
There are two types of cracking:
Catalytic Cracking - High temperature and the Zeolite catalyst
Thermal Cracking - Extremely high temperature and pressure only
Mechanism
The mechanism is not required but simply due to the high temperature, the bonds break homolytically to form carbon free radicals. So they quickly form an alkene or an alkane by bonding to the nearest carbon atom
Uses of Cracking
The Main use is to obtain high demand shorter chains of alkanes such as octane
It is also used to obtain alkenes such as ethene for the production of the polymer polyethene which is very profitable
Used to produce hydrogen in some rare cases
