Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsHalogenoalkanes
When one or more hydrogen atoms of an alkane are replaced by halogens
A good example of a halogenalkane is chloromethane where the hydrogen is replaced by a chlorine atom
You will need to know the structure of the halogenoalkanes. It is very similar to the structure of carbocations and the alcohols
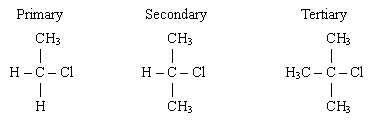
The Primary halogenoalkane has a single R groups and 2 hydrogen atoms. This is considered to be the least stable
The Secondary halogenoalkane has two R groups and 1 hydrogen atom
The Tertiary halogenoalkane has 3 R groups attached. This is considered to be the most stable
An R group can be either an alky group or a long chain of carbon atoms. Remember that we consider the carbon atom that is directlty bonded to the halogen
Identifying the type of halogenoalkane is very important especially when understanding the rate of the reaction
When different halogens are attached to the alkane we name it depending on the halogen:
1. Chloroalkane
2. Bromoalkane
3. Iodoalkane
Boiling and Melting Points
Halogenoalkanes have very low boiling and melting points and they are very volatile. That is why when we heat them in reactions it must be heated under reflux to prevent the halogenoalkane escaping
The boiling point of the halogenoalkane from fluorine to iodine must increase due to their increase in induced dipole forces. This is because, the number of electrons increases
Solubility
Halogenoalkanes are not soluble in water or they are slightly soluble. This is mainly because the halogenoalkane has mostly non polar bonds so the energy released from dissolve would not be negative. However, they are very soluble in organic compounds
Reactivity of Halogeonalkanes
Halogens are more electronegative than carbon atoms and so they will be partially negative charged and the carbon atom will be positively charged. This makes the carbon atom easily attacked by nucleophiles or neagtive ions. It is a must for you to show this dipole charges in you mechanisms!
The more electronegative the halogen atom is, the more polar the bond and greater positive charge on the carbon atom. So this will make the reaction much faster but, the reaction also depends on the bond ethalpy of the carbon to halogen bond. This is because this is where the reaction occurs and for the reaction to occur the halogen atom must be broken away from the molecule. For this very reason, Iodocompounds reacts much more faster than Fluro or ChloroCompounds.
We have discussed this before where we used to see the presence of these halide ions using silver nitrate we can see that Iodocompounds will make the precipitate first, then bromocompound, then Chlorocompound and finally fluorocompounds
Iodocompounds > Bromocompounds > Chlorocompounds > Fluorocompounds
This is many due to the bond ehthalpy and the activation energy of the reaction. The Fluorine - Carbon bonds are stronger than Iodine - Carbon bonds. So the reaction is much slower.
The same could be applied for Halogenalkanes!
Reactions of Halogenoalkanes
These are the reactions in which halogenoalkanes can undergo. You will need to know the conditions and the products for each reaction. Remember that all of these below reactions are nucleophillic substitution reactions
Hydrolysis of halogenoalkanes is usually done using concentrated sodium hydroxide or potassium hydroxide under reflux and this produces an alcohol
CH3Cl + NaOH → CH3OH + NaCl
What happens here is that the chlorine atom is replaced by an OH group so this is called a substitution reaction. The mechanism is nucleophillic
The conditions are concentrated NaOH and heating under reflux
The same could also occur with pure water as water can act as a nucleophile but the rate is very slow and takes a long time
When halogenoalkanes are mixed with Concentrated ammonia dissolved in an alcoholic medium and heated under reflux then we will get an amine
CH3Cl + NH3 → CH3NH2 + HCl
The concept is very similar to hydrolysis or the substitution by the OH group. Instead here the new group added is the amine group NH2
Ammonia acts as a nucleophile just as water does but ammonia is a much stronger nucleophile. The conditions for this reaction is that we use concentrated ammonia in PURE alcoholic medium heated under reflux
For this reaction we use potassium or sodium cyanide dissolved in pure alcoholic medium heated under reflux to get a nitrile
CH3Cl + KCN → CH3CN+ HCl
The best thing about this is that the carbon chain has increased leading to more carbon atoms. This can be used to obtain compounds of higher carbon atoms. So remember this because this reaction is usually the intermediate or the middle of a larger reaction. For example, if we need to make propanoic acid from ethanoic acid this reaction is needed
The conditions needed are that concentrated KCN must be dissolved in pure alcoholic medium heated under the reflux. The reason why CN- is a good nucleophile is because it is a negative ion!
Halogenoakanes can undergo elimination when it is reacted with NaOH/KOH dissolved in pure alcoholic medium heated under reflux. The only difference between hydrolysis and this reaction are the conditions. We need to use concentrated NaOH and we need to use hot ethanol as the alcoholic medium. This reaction will cause an HCl molecule to be released and also form an alkene
C2H5Cl → CH2H4 + HCl
This is almost similar to the dehydration of alcohols but in this case a hydrogen and a halogen atom is removed so not a water molecule and so we will get HCl
This reaction is called an elimination reaction and this only occurs when the required conditions are met. For this reaction, you don't need to know the mechanism
General Mechanism
This particular mechanism can be applied to the above reactions as they all react almost the same way except water and ammonia
The pathway in which the reaction takes really depends on the stucture of the halogenoalkane. There are two mechanisms in which the reaction can take place depending on the structure and that is:
This mechanism only occurs when the halogenoalkane is a tertiary halogenoalkane. The name means that it is a nucleophillic reaction step 1. This means that the rate of the reaction depends on the 1st step - the ionisation of the halogenoalkane
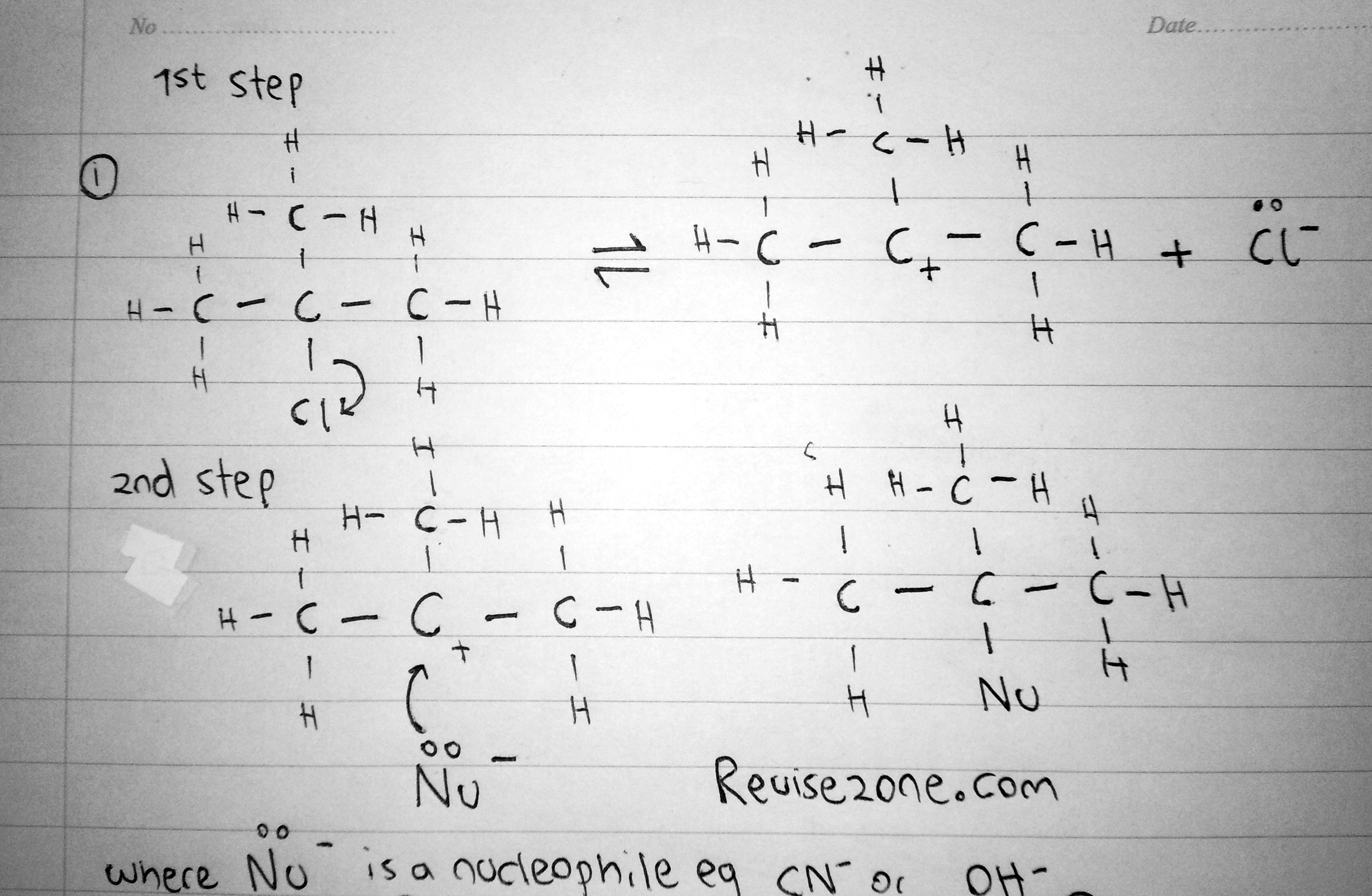
The central carbon atom of the halogenoalkane is surrounded by many R groups around it. So the nucleophile can not attack the partially positive carbon atom inside. This is called steric hindrance. In other words, there is a blocking effect which prevents the nucleophile attacking the carbon atom. So for the nucleophile to react it must wait until the Halogenoalkane ionises to form a carbocation
C2H5Cl → C2H5+ + Cl-
This is a reversible reaction and this happens very slowly and rarely. That is why the rate mainly depends on this step
Once the carbocation is formed, the nucleophile will give its lone pair to the carbocation to form the new product. This happens very quickly
The reason why the carbocation is able to exist is because it is teritary carbocation so it is highly stable. So for a short while it can exist in nature due to the positive inductive effect of the R groups in the carbocation
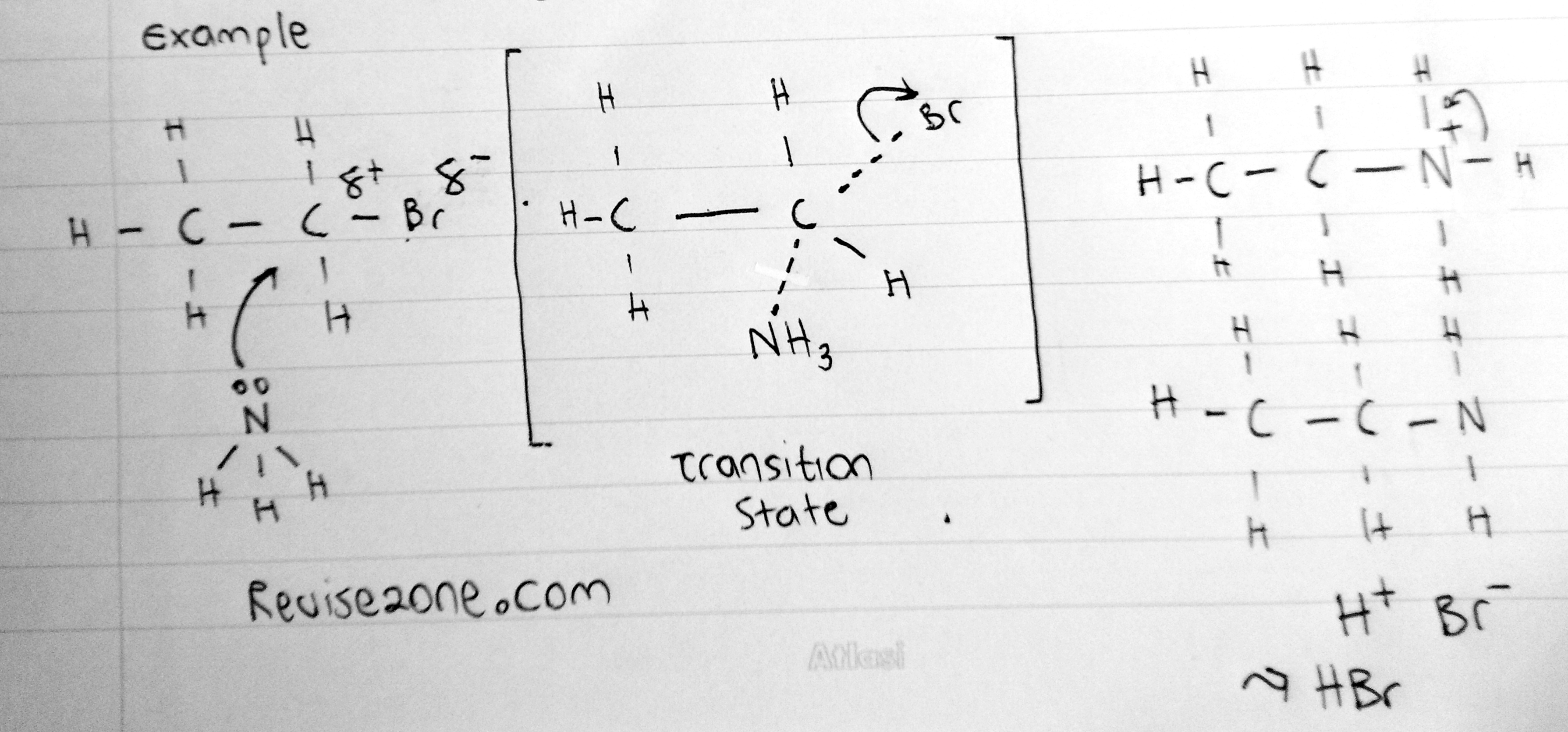
This is for primary halogenoalkanes
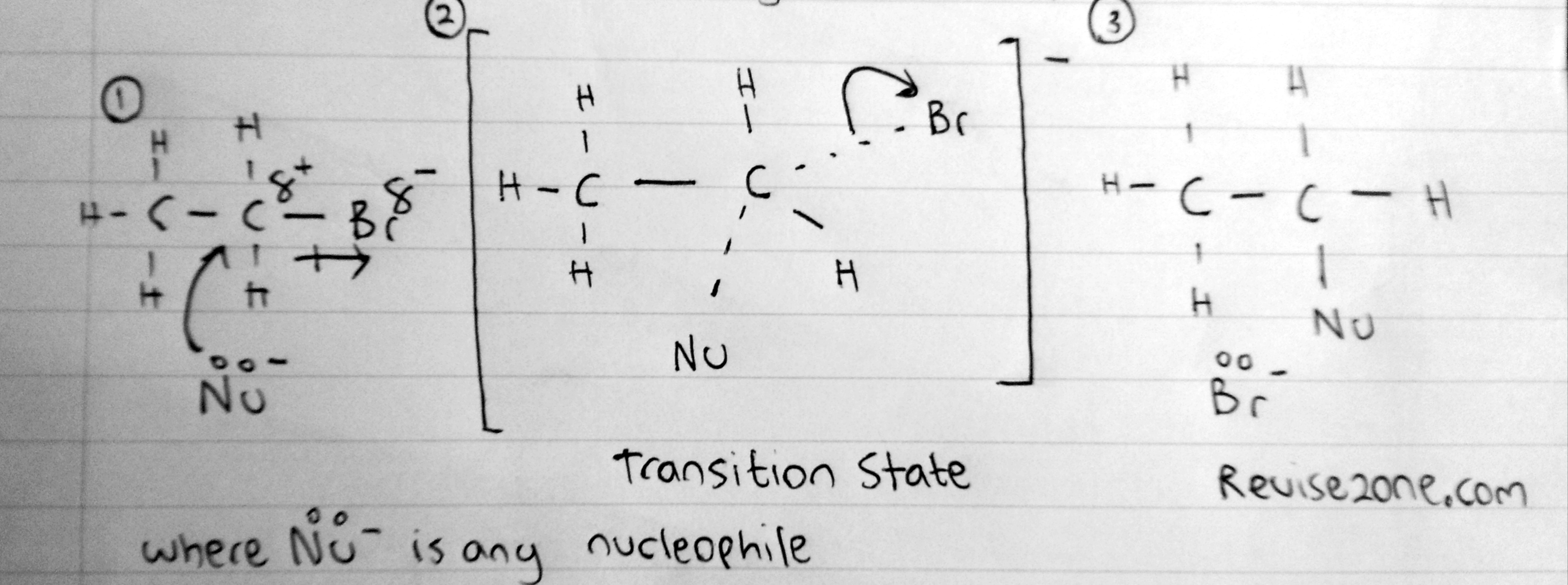
No carbocation is formed because primary carbocations are highly unstable so it can not exist. So what really happens is that the nucleophile donates its lone pair to the partially positive carbon atom while it is still attached to the halogen. This is considered to be a half broken bond and so there will be a dotted line between the Carbon and nucleophile and between the Carbon and Halogen at the same time. This is known as the transition state and it can not exist in nature. So immediately the Carbon - Halogen Bond breaks heterolytically to form a Chlorine ion which will react with the H+ ion to form HCl
We draw this transition state seperately and we draw the molecule in a bracker to show it is an ion formed. Also we need to show the half broken bonds as dotted lines. The nucleophile must be on the opposite side on the halogen because the halogen will repel the nucleophile
Secondary Halogenoalkanes can either take the SN1 or the SN2 mechanism
What about for Water & Ammonia?
For water and ammonia, the nucleophiles may have to take one more step so we will see that below
Requirement for Diagrams
You need to show the bonds properly and the dotted bonds also. You also must show the dipole and the partial charges on the Carbon to halogen bond as this is what shows the reason why the nucleophile attacks
Also the full arrows must be either from the middle of the bond or from a lone pair or else the examiner may penalise your answers
Uses of Halogenoalkanes and Halogenoalkenes
There is a variety of uses of halogenalkanes and halogenoalkenes such as making polymers like polytetrafluroethene and PVC. We will see the main uses below:
CFCs were considered to be safe as they were inert, unreactive and non toxic and inflammable. They were used in aerosal sprays, refrigerant, coolants and for bloating polystyrene.
Fortunately, CFCs are banned because they were damaging the Ozone layer and also slighlty causing an increase in global warming. The reason why it damaged the Ozone is that due to the presence of UV light at high altitude the chlorine atom will break from the CFC homolytically to form chlorine free radicals ( Discussed before ). This free radical will attack the Ozone O3 to make Cl2O molecule. This causes a chain reaction as more free radicals will be formed and will attack more ozone molecules. Ultimately, this will cause a hole in the ozone layer and let harmful UV light to pass
To prevent this, we use the substitution Hydrofluorocarbons or HydroChloroFluoroCarbons. HFC does not have any chlorine atoms so it has no risk to the Ozone layer. HCFC has chlorine atoms but it quickly breaks down at a lower altitude before reaching the ozone . HCFC has some risks but it is at minimal. Another famous substitute used for CFC is alkane such as butane gas. If you go look at your deodorant spray, it will say it has butane in it and so it is flammable. This is actually one of the problems of using alkane gas as the substitute because it can cause fires. Also the butane gas can react with nitrogen oxide in air to form photochemical smog which is another problem
It is used to make useful plastics such as Teflon and PVC which are good insulators and very durable. The main problem arises from disposing them as they take space and releases very harmful gases such as HCl when burnt
As halogenoalkanes are insoluble in water and very volatile they can be used as solvents for dyes