Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsAlkenes
They are Unsaturated Hydrocarbons with the General Formula CnH2n
This is a structure of Propene:
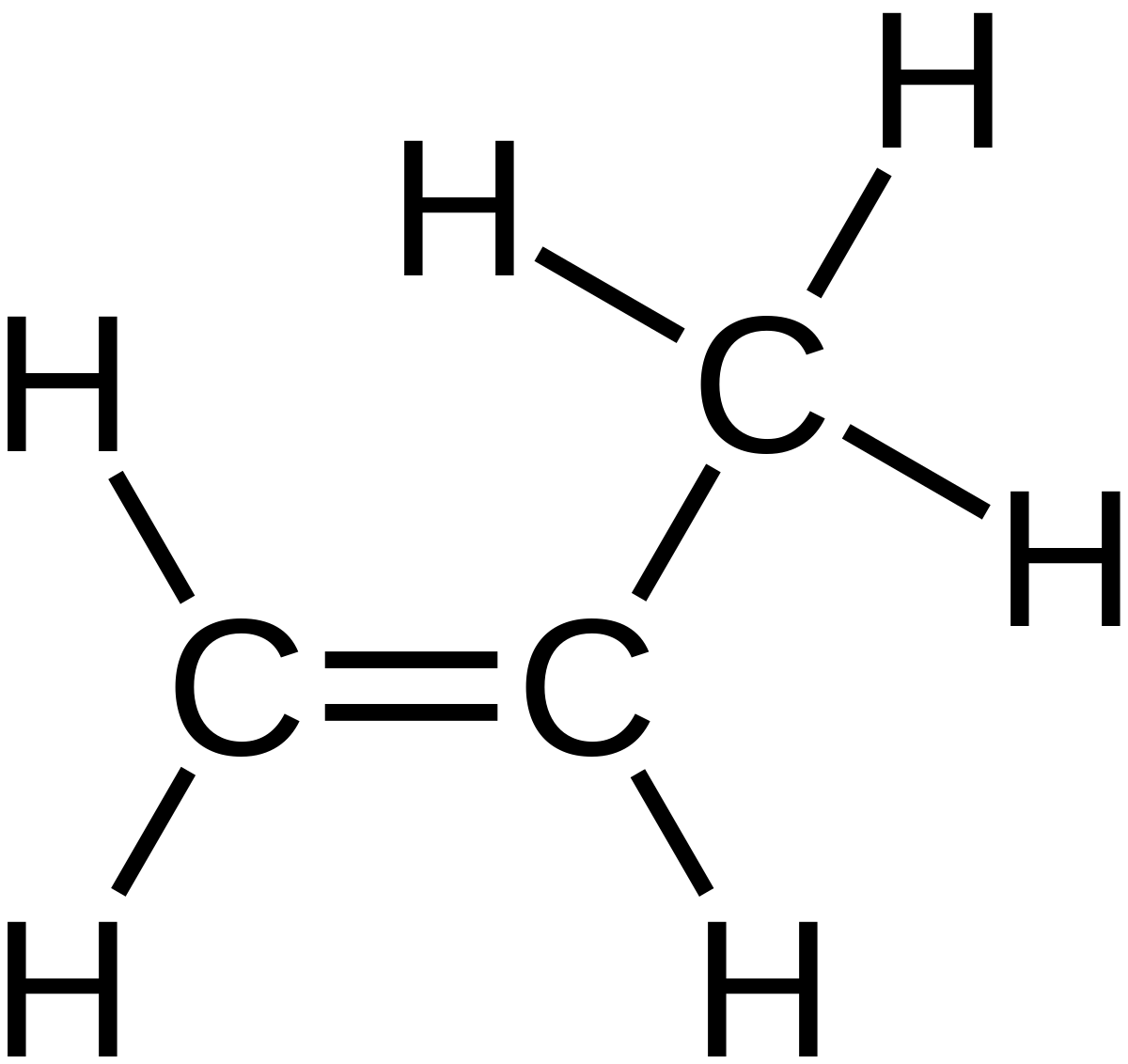
These are called unsaturated hydrocarbons because they have atleast one carbon to carbon double bond. This makes the substance very reactive due to the high density of electrons in the double bond. This acts as an nucleophile and is easily attacked by electrophiles. In fact, most of the reactions of alkenes are called electrophillic addition
Due to the carbon double bond, it makes the region slightly negative charged and so electrophiles are attracted to it
But before we talk about the mechanisms and reactions, we need to know what an electrophile and nucleophile is
Electrophile
An atom or an ion that is partially positive charged and accepts a pair of electron
The common examples are carbocations, H+ or partially positive charged atoms δ
Nucleophile
Substance that donates a pair of electron
Good examples are negatively charged ions or carbon double bonds or even atoms with lone pairs
A common example is Ammonia
Reactivity of Alkenes
Like we said the reactivity of alkenes depends mainly on the carbon to carbon double bond. As it is easily attacked by electrophiles. Also the 2nd bond in the double bond is weaker than the first ( π bond is weaker than the sigma bond ). So they usually break easily to form new bonds
Most of the reactions of alkenes are called electrophillic addition. This is because the mechanism is due to the electrophiles attacking the C=C bond and the reaction is an addition reaction. If you want to know the types of organic reaction we recommend you to visit the previous chapter
Normal Reactions of Alkenes
We will need to know some of the reaction mechanisms but, not for all reactions. In this part, we will discuss the basic reactions which you only need to know the equation and products but not the mechanisms
There is incomplete and Complete combustion. You will need to know the products for each reaction so we will take ethene as the example
When Ethene react in excess oxygen (Complete Combustion):
C2H4 + 3O2 → 2CO2 + 2H2O
When Ethene is burnt in limited oxygen, it has several different equations:
C2H4 + 2O2 → 2CO+ 2H2O
C2H4 + O2 → 2C+ 2H2O
The reason why I have given two equations is that sometimes the questions may ask the production of a black substance only which is carbon ( or soot )
But the general equation is the first one
This is a more slower way of oxidising alkenes and we use either Hot Concentrated acidified KMnO4 or Dilute Cold acidified KMnO4. Each of these conditions will result in different products
Hot Concentrate Acidified KMnO4
Due to the high temperature, the carbon double bond breaks or splits resulting into two seperate molecules. Then Oxygen atom bonds with these carbon atoms so we get a C=O group in each seperate molecule. The products also depends on the position of the double bond in the alkene
The below diagrams will summarise all the possible products
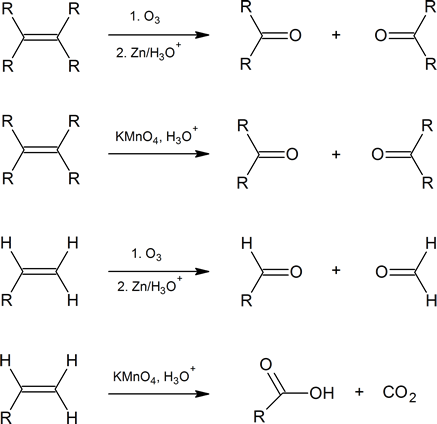
To simply put it, if the double bond is attached to the 1st carbon atom. Then the products must give out CO2 & H2O and some other products
The understanding is very simple. What you have to do is to split the alkene at the double bond which results two molecules then bond a oxygen atom. This could either create carbon dioxide, water, ketones or an aldehyde
You don't need to know what are ketones or aldehydes right now but, remember they are carbonyl coumpounds with the C=O group
Dilute Cold Acidified KMnO4
In this the π bond in the carbon double bond breaks ( this does not split or fully break ) and this bonds with and OH group. It is very similar to an alkane instead the the hydrogen atoms are OH groups. Also keep in mind that as the double bond breaks. This leaves two carbons not bonded so two groups of OH groups will attach to form a diol
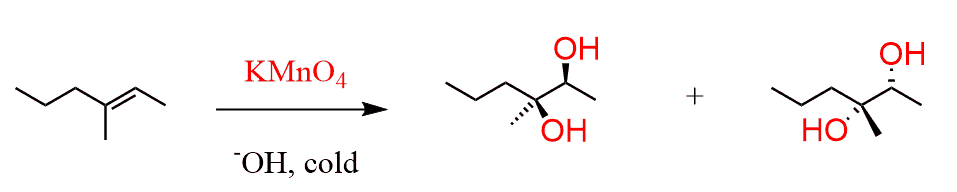
Electrophillic Addition
For these addition reactions, the mechanisms are required
All of these below reactions are addition reaction which means only a single product is formed. These reactions involve the addition of:
1. Hydrogen
2. Water/Steam
3. Halogen
4. Hydrogen Halide
We will discuss each one in depth now:
We will take ethene as the example:
C2H4 +H2 → C2H6
So during the reaction the double bond breaks and the two hydrogen atoms will bond to the carbon atoms
The below is the mechanism of the reaction. Make sure you remember how to draw this:
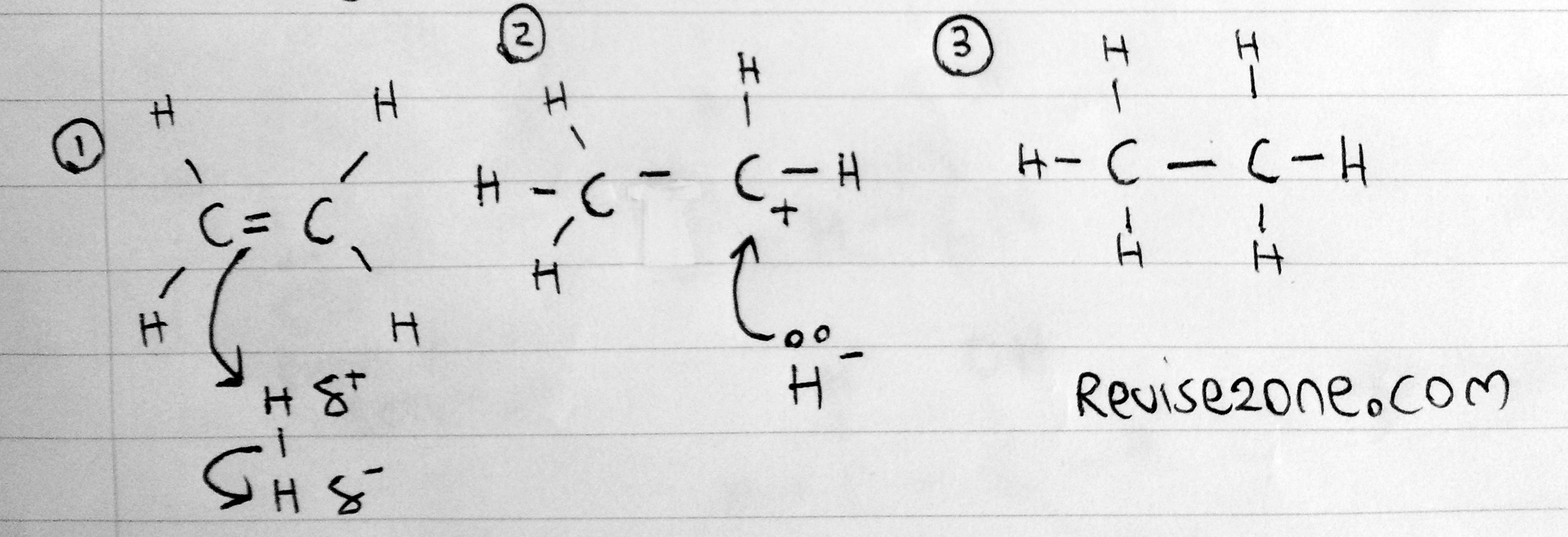
Catalyst: Heated Nickel
Temperature: 150°C
But the main problem is locating the electrophile. Hydrogen is non polar so why will it attack the double bond. This is because, when the hydrogen molecule gets close to the high electron density carbon double bond, a partial positive charge is INDUCED on one of the hydrogen atoms. This is the same for other molecules such as bromine
When the water molecule reacts and give its OH group to the alkene to form an alcohol
C2H4 +H2O → C2H5OH
This is the mechanism of the reaction in this case the lone pair of the water molecule acts as the electrophile
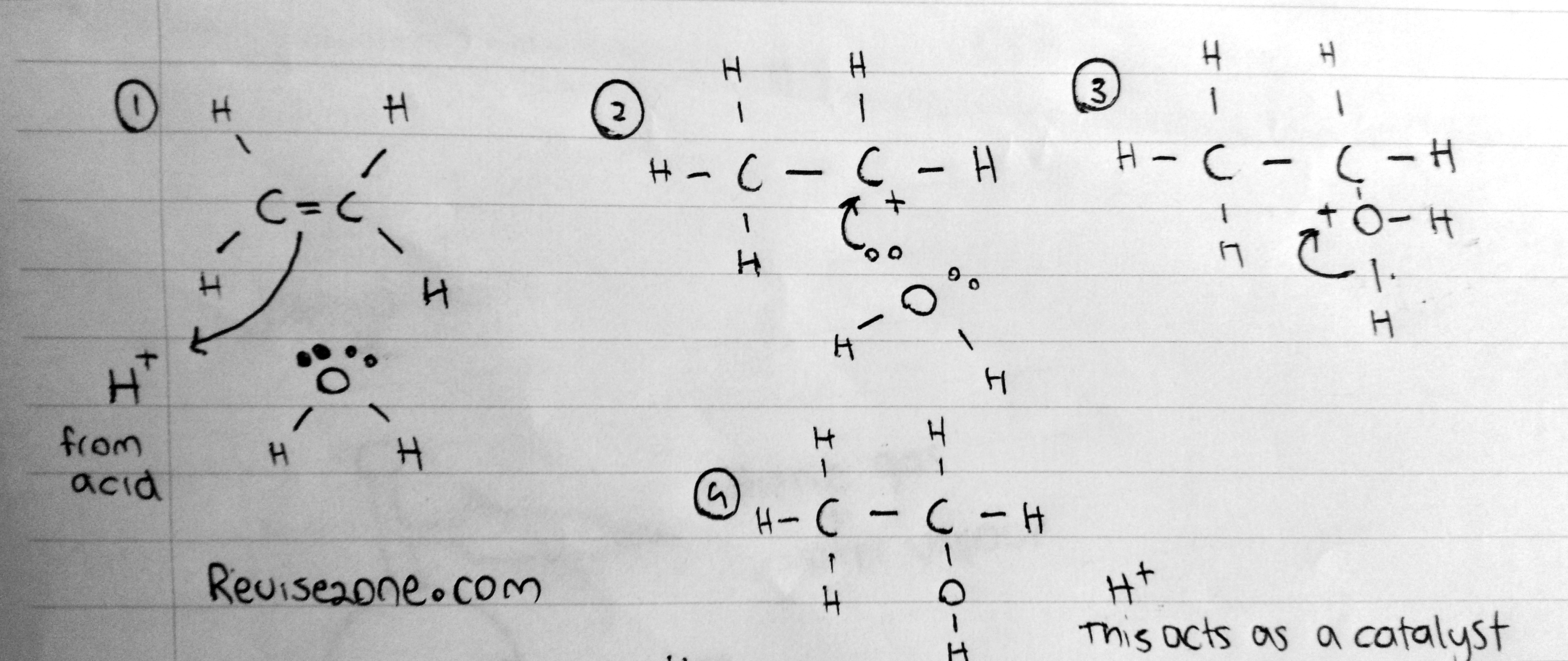
Catalyst: Phosphoric acid
Temperature: 300°C
Pressure: 60atm or 6 Million Pascals
This is very similar to addition of hydrogen but instead this is two bromine atoms
C2H4 +Br2 → C2H4Br2
The mechanism is similar to the hydrogen but we use bromine instead. Also this could be applied for any other halogen in the same way
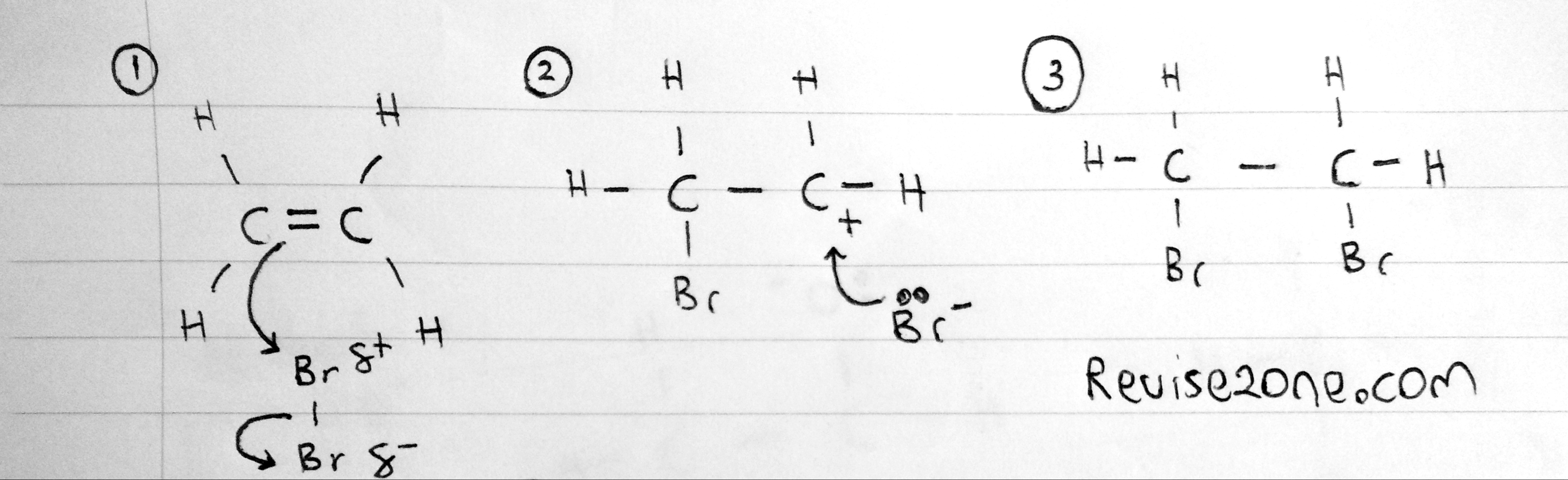
This reaction can occur at room temperature and is used as a test for alkene or unsaturated compounds because the bromine liquid / water decolorises
This seems more obvious as the molecule is certainly polar and so the Halide atom will be partially negative charged and act as an electrophile
C2H4 + HBr → C2H5Br
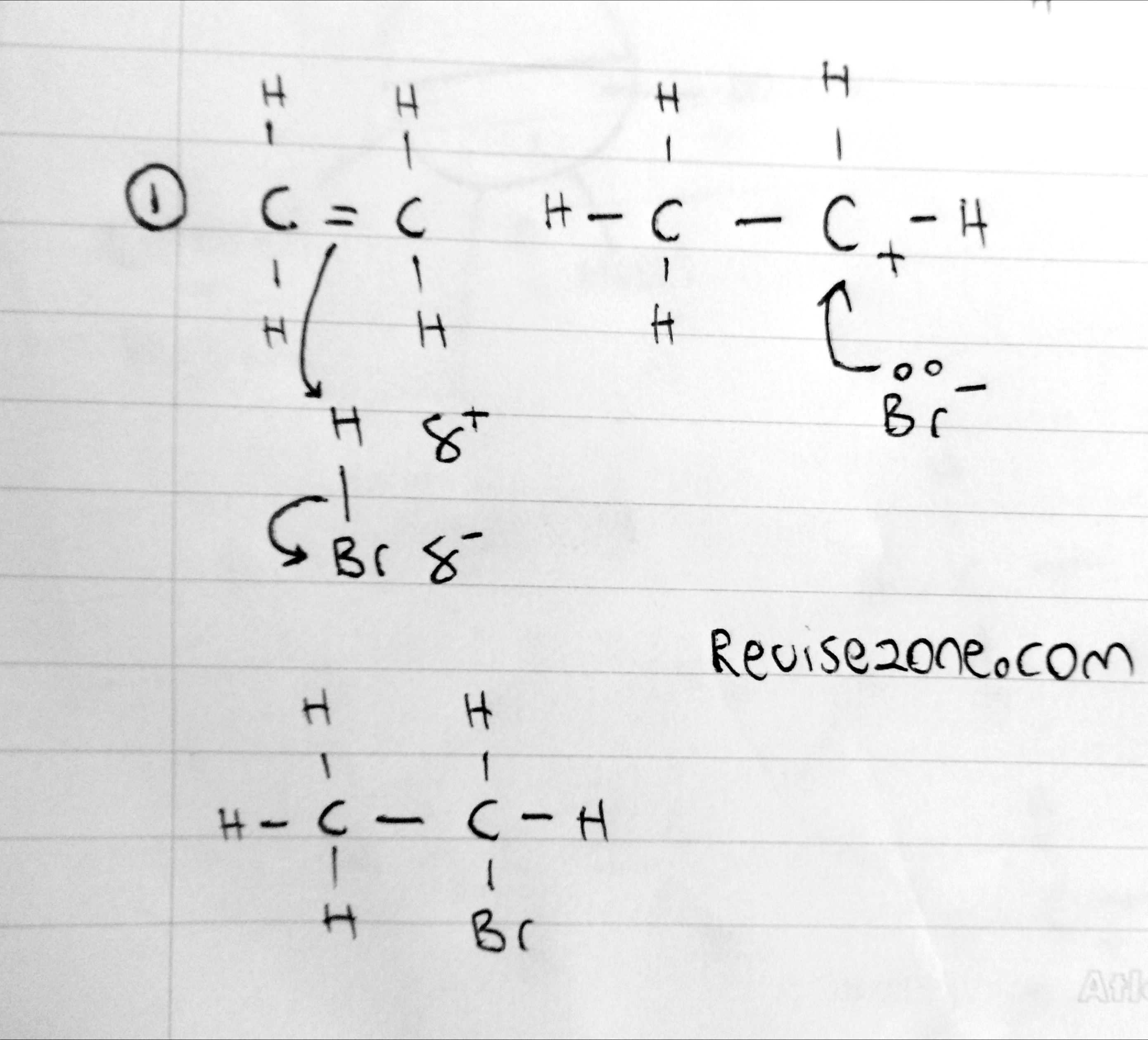
This is also very similar to addition of bromine gas but in this case there is only a hydrogen and bromine atom.
Reaction also occurs at room temperature
Notes and Reminders
You need to know that the mechanisms are very important and we use full curly arrow to show the movement of the electrons
The key to getting high marks is to follow the rules properly. We need to start the arrow either from the middle of the bond or from a lone pair. This is a must because these arrows show the movement of arrows
Remember to label the dipole charges and moments on the molecules
We use full arrows to show it is a hetrolytic fission and not homolytic fission
Stability Rule
Using Markovnikov's rule we can predict the possible products that will have the higher concentration depending on its stability. Here is an example:
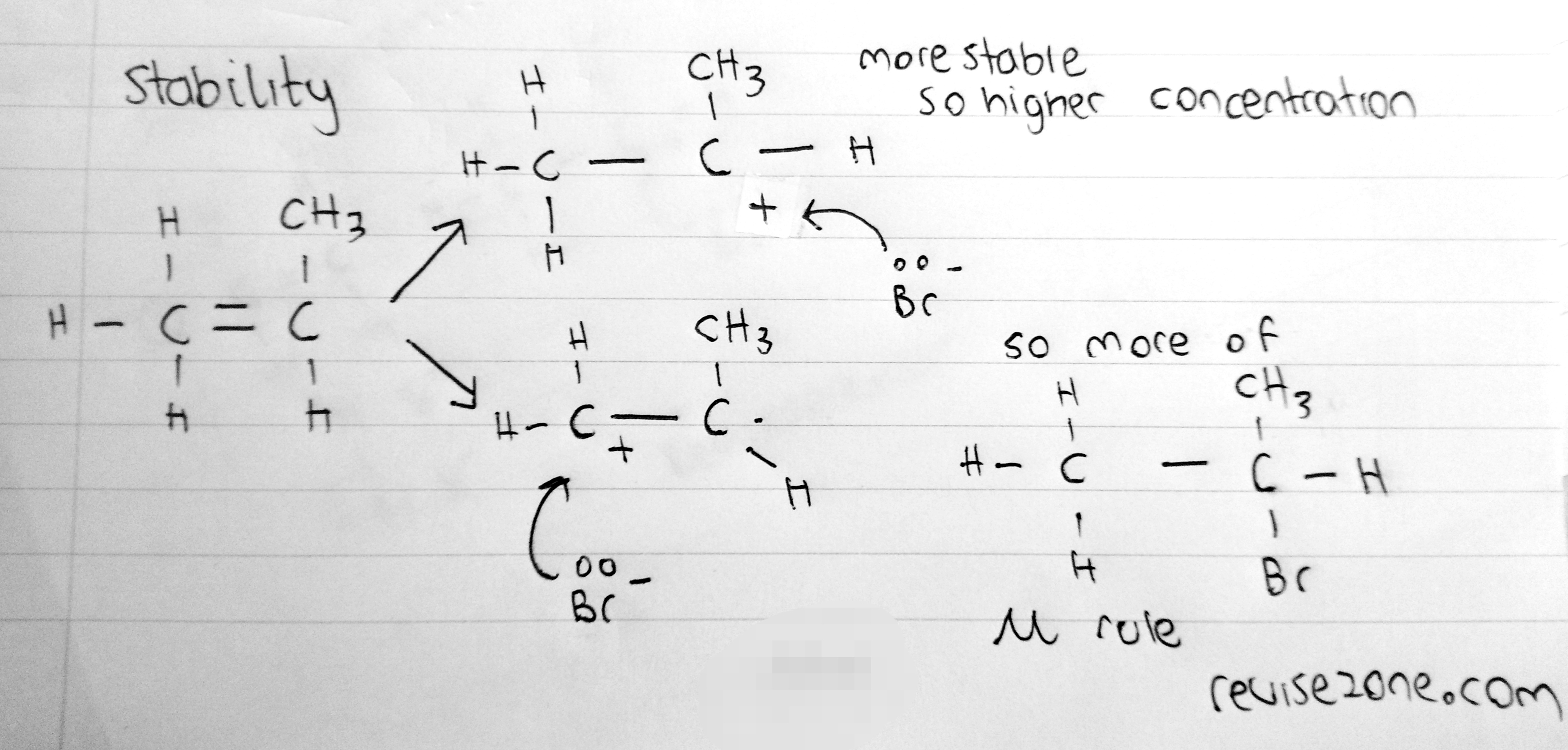
So the carbocation that is the most stable will be more abundant and have a higher concentration. This is not visible with bromine or hydrogen molecules only but with molecules of different atoms. This is because, in this case the atoms may rearrange in different orders
