Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsIntermolecular Attraction Forces & Solubility
Intermolecular forces are attraction forces between molecules. They are still called bonds but they are not Covalent bonding
Atoms within a molecule are covalently bonded but, molecules are bonded using intermolecular forces
We will see the 3 types of intermolecular forces:
- Induced dipole Induced Dipole forces
- Permanent Dipole forces
- Hydrogen Bonding
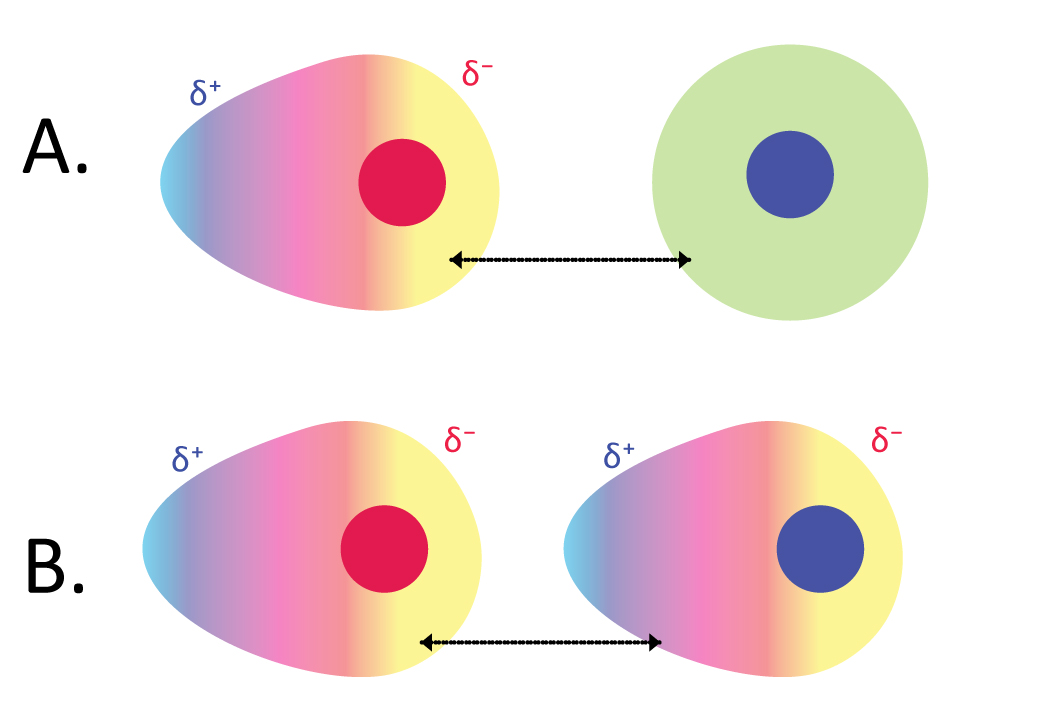
This is also called Van der waal forces and temorary dipole forces. This is the weakest intermolecular force and it is present in every atom and molecular structure.
This arises due to the idea of probability. For example, if we take a neutral atom, we know the electrons are orbiting around the nucleus of the atom but, if more electrons happens to be more on one side of the atom this causes one side of the atom to be partially negatively charged and the other side partially positive charged
When one molecule and atom is like this, it induces a charge on the neighbouring molecules and atoms causing a chain reaction. This then has weak induced dipole forces
What you need to know from this is that, the more electrons the molecule or atom has the greater the induced dipole force (as higher probability and higher induced charge)
Also if there are more contact points and large surface area of the molecule then it will have stronger induced dipole forces. This is especially important when the questions ask you to explain why the boiling point of some isomers of the same molecule has a lower boiling point. This is because due to the decrease in surface area or contact points
For you to understand this we need to know a new term - Electronegativity
Electronegativity is the ability of an atom to pull a pair of valence electron from a bond to itself
This definition is very important and you need to understand what it means. When an atom is more electronegative than the other atom it means the electron pair is more closer to it
For example, the hydrogen chloride gas ( HCL ) has a single electron bond pair. Chlorine is said to be more electronegative than Hydrogen, which means the bond pair is more closer to the chlorine atom than the hydrogen atom. This means that one side of the partially negative (electronegative side) and one side is partially positive
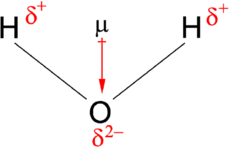
Make sure you label the partially positive side and the partially negative side. Also label the dipole moment direction
We will show a labelled diagram of a water molecule:
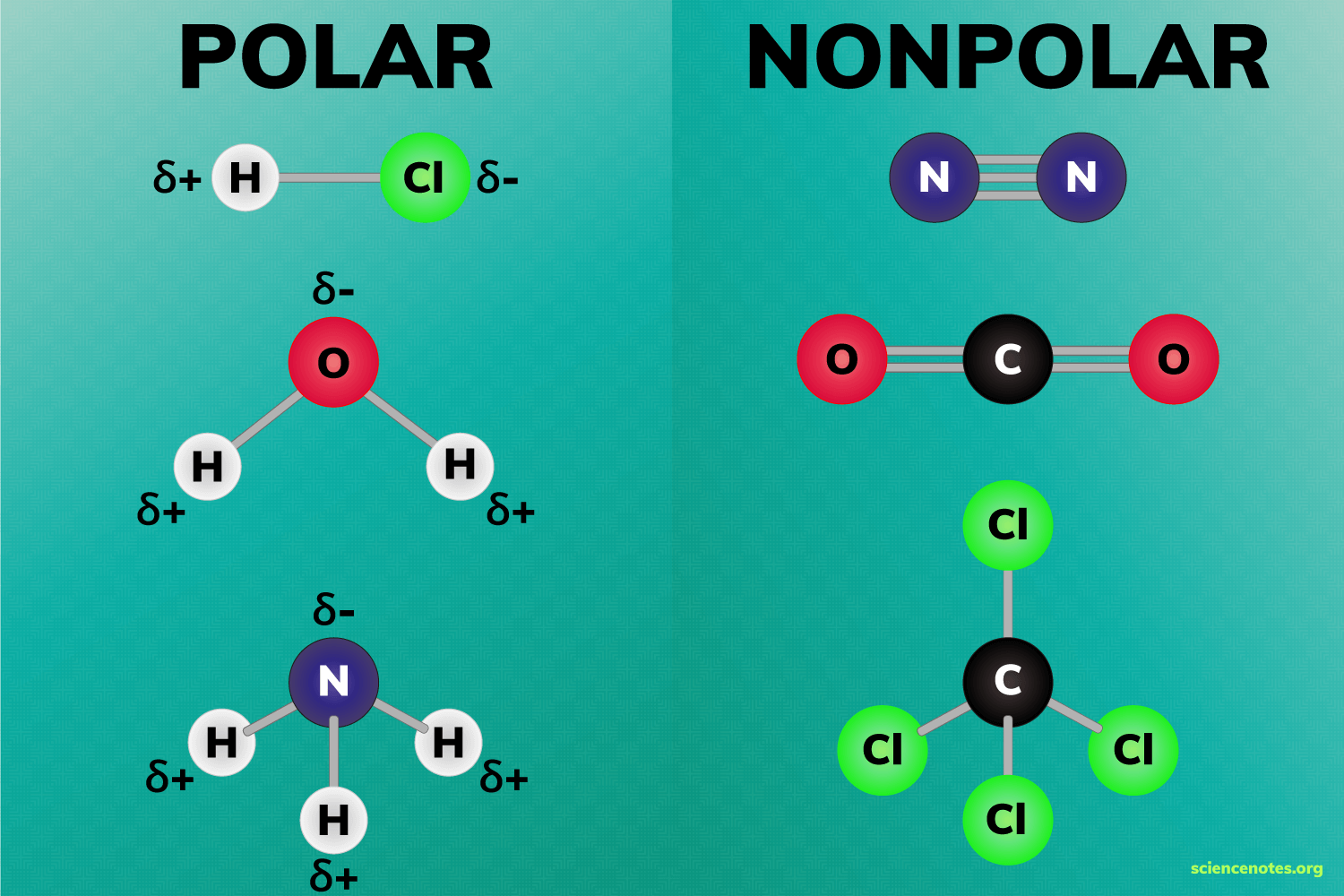
Always remember that Polar bonds are formed when there is a difference in electronegativity between the two atoms. Which means that the bond between two identical atoms are said to be non polar. For example N2 is said to be non polar. Also Carbon and Hydrogen atoms have almost identical electronegativity which means the bonds are also said to be non polar. For example, the methane molecule is said to be non polar
Polar bonds depends on the difference on the electronegativity (Higher the electronegativity, Higher the polarity of the bond) but, Polarity of the molecules depends on two factors:
1. Polarity of the bond
2. Shape of the molecule
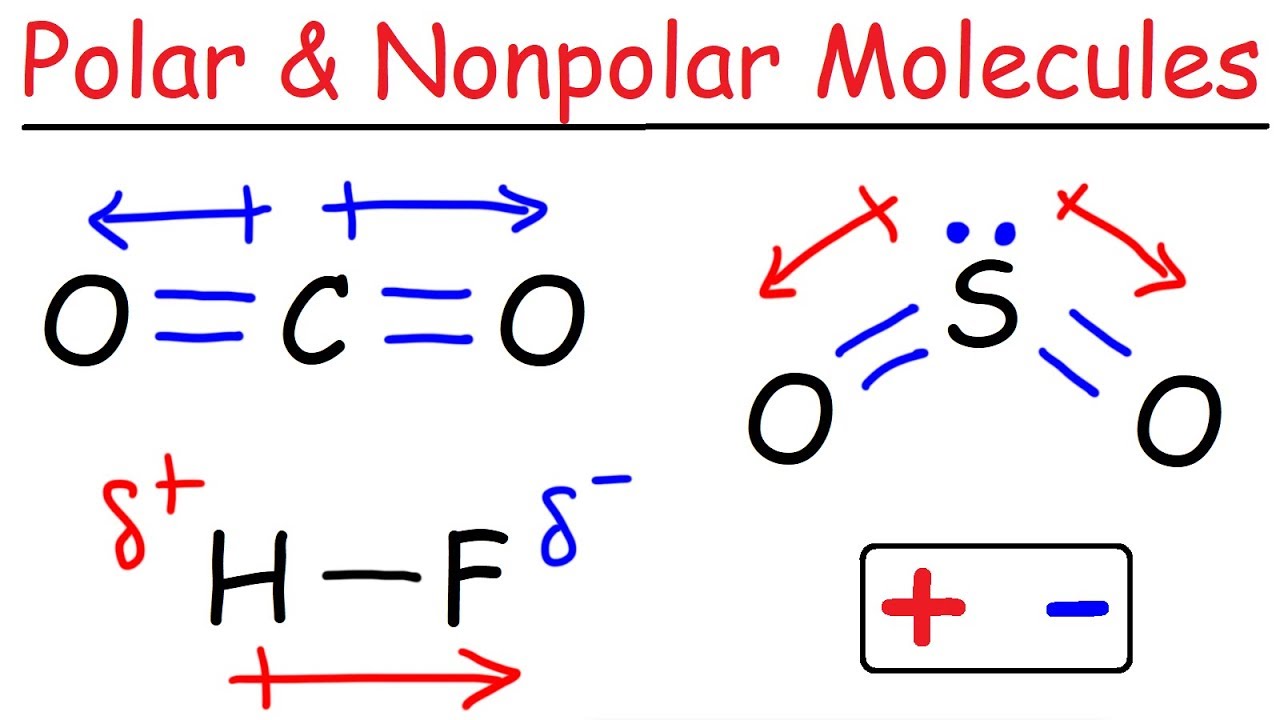
When the shape of the molecule is arranged in a way that cancels out the dipole of the polar bonds ( For example, carbon dioxide contains polar bonds but, due to its linear structure, the dipole moments cancel out. So Carbon dioxide is an non polar molecule that has polar bond (this means it has permanent dipole forces)
Understand, that this seems very similar to induced dipole forces but, this remains permanent as the names state and is way stronger than the induced dipole forces
So for permanent dipole forces to occur there MUST be polar bonds in the molecules. Polar Molecules are not a must but are more likely
We will take the example Carbon dioxide and other molecules:
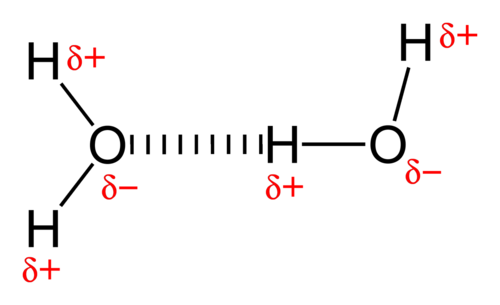
The definition of this bonding is compulsory for you to know
It is the attraction forces between a hydrogen bonded to a very electronegative atom and a very electronegative atom
For hydrogen bonding to occur there must be two conditions:
1. Lone pairs present
2. Hydrogen bonded to a very electronegative atom ( Either Oxygen, Nitrogen or Flourine ONLY )
Notice this only works when the electronegative atom is either Oxygen, Nitrogen or Flourine
Also hydrogen bonding are relatively stronger than any type of intermolecular forces but, its strength mainly depends on the lone pair to hygrogen bonding ratio. That is why water has a higher melting point than HF
More on electronegativity
You do not need to know for now the trends of electronegativity (This will be covered under periodicity) but, remember that only Nitrogen or Oxygen or Floruine undergoes Hydrogen bonding( NOF ).
How to answer a question
Usually the question will be related to something about melting or boiling point of a substance
First State the type of intermolecular force(s) and make sure you say it's weak
Discuss the strength of the IMF force compared to the other types. For example, Hydrogen bonding is the prominent force which is the strongest IMF and so it requires more energy
Then state that due to weak intermolecular forces, there is weak bonds formed which require very little energy
Solubility
Solubility measure the amount of moles or mass a solute can dissolve in a solvent
You need to know what makes a solid soluble and what reasons suggests why some solids are insoluble
The idea you must realise is that for anything even for reactions to occur, it must be energy profitable (or energy must be released). In other words, the overall reaction must be exothermic.
If we take the solute Sodium Chloride and dissolve it in water. What really happens is that the energy is formed when water and ions(sodium and chlorine) bonds are formed, which is enough to break the strong hydrogen bonding between molecules. This is a very simple idea and helps us predict that the stronger the ion-water bond, the higher chance it dissolves. Let us take a different example, Barium sulfate is insoluble in water because, the energy released from forming Barium ions and water bonds or Sulfate ions - water bonds is less than the energy needed to break the hydrogen bonding between water molecules or the ionic bonding between the Barium and Chlorine ions. That's why we have learnt that Some salts are insoluble due to their strong Ionic bonding
Salts don't neccesarily need to release energy in water, some could be endothermic but, this goes against with our point that only exothermic reactions can occur. For now, you don't need to know why it is possible and it will be covered under A2 Entropy
The idea that usually only Polar molecules dissolve in Polar Molecules only and Non polar molecules dissolve in Non polar molecules is from the basis of the idea that the molecule - water bond must be stronger and release more energy than the strong hydrogen bondings between water molecules.
