Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsIonisation Energies & Basic Periodicity
You will need to know how to define Ionisation energy
Defining Ionisation Energy (IE)
The energy needed to remove one mole of electron from one mole of gaseous atoms or cations to form one mole of cations
There is an alternate definition:
The energy needed to remove an electron from every single gaseous atom or cation in mole of a sample of the gaseous atoms or cations
Ionisation energy is the energy required so we need to spend energy to remove an electron from its orbital. Why? This is because, the electrons are strongly held by strong electrostatic forces between the nucleus and the electrons
For this reason, the Ionisation energy is always positive / endothermic
The term ionisation energy is only for gaseous atoms only
Ionisation energy is a very general category. We will now define some specifics of Ionisation energies such as the first and the second ionisation energies
The First Ionisation energy (IE1)
The energy required to remove one mole of electron from one mole of gaseous atoms to form one mole of gaseous unipositive(+1) ions
X(g) → X(g)+ + e-
This is the representation of the first ionisation energy in the form of an equation
Second Ionisation Energy (IE2)
The energy required to remove one mole of electron from one mole of gaseous unipositive ions to form one mole of gaseous dipositive cations
X(g)+ → X(g)2+ + e-
A misconception is that students think that the Second ionisation energy is the energy needed to remove electrons to form a dipositive cation from a neutral atom. This is not correct and in fact this is equivalent to the sum of both the first and the second ionisation energies
X(g) → X(g)2+ + 2e- (1) X(g) → X(g)+ + e- (2) X(g)+ → X(g)2+ + e- (3) 1 = 2 + 3
Factors affecting Ionisation Energy
There are 4 Factors which affect the ionisation energy of an element
1. Nucleur charge
2. Atomic Radius
3. Shielding Effect
4. Spin-pair repulsion
We need to know how each of these factors affect the ionisation energies
When the nucleur charge increases, the proton number is increased so there is greater electrostatic attraction forces between the nucleus and the valence electrons and so more energy is needed to remove the electrons. So the ionisation energy increases when the nucleur charge or proton number increases
IE ∝ Nucleur Charge
When the atomic radius increases the seperation between the electrons and nucleus increases and so there is weaker electrostatic forces and so the ionisation energy decreases
IE ∝ 1/Atomic radius
In other words, the ionisation energy is inversely proportional to the atomic radius
When the number of inner core electrons increases, the shielding effect increases. When the shielding effect increases then the effective nucleur charge or attraction forces is less than normal. So the ionisation energy decreases
IE ∝ shielding effect
Let us define what is shielding effect:
Shielding effect is the reduction in the effective nucleur charge due to the difference in attraction forces on the electrons by the nuclues and the repulsion forces by the inner electrons
In other words, the inner core electrons repel the outer electrons and so the resultant attractive force experienced by the valence electrons decreases
Trends in Ionisation Energies
We will see the trend of the first ionisations(general ionisation energy) of elements across the period and also down the group, This is more of an periodicity note so for more periodicity notes go to our Periodicity chapter
For either trend, just follow what we have written. It is enough to score full marks as it is extracted from mark shemes
Ionisation Energies across the period
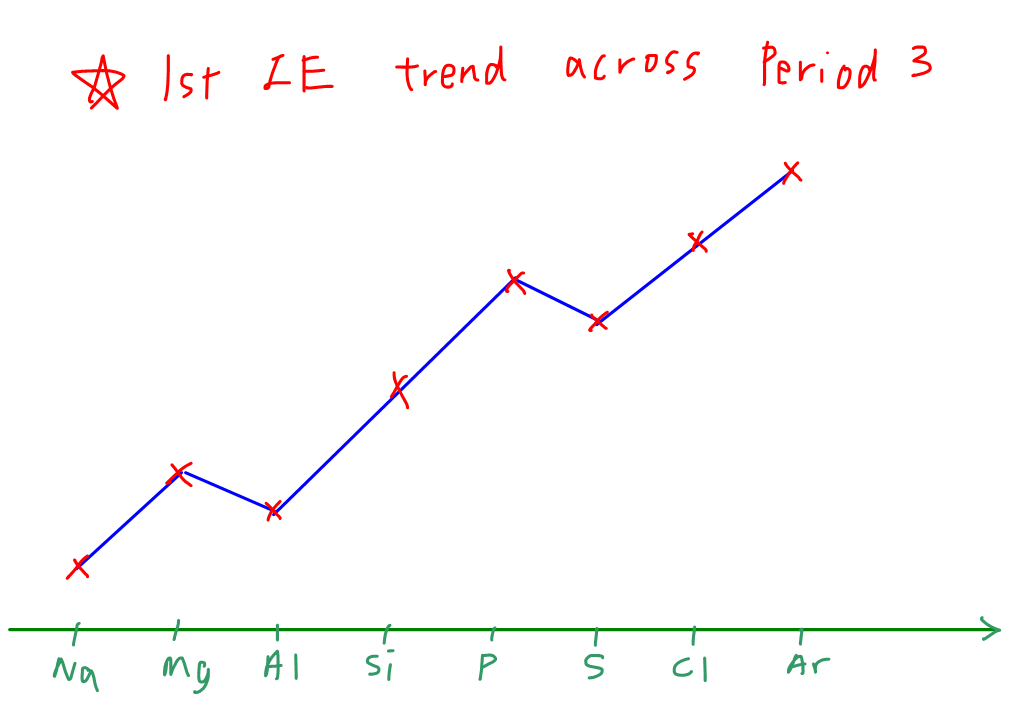
Across the period, the Ionisation energies generally increases. This is because, the shielding effect is more or less the same as electrons are added to the same principle Quantum shell and the atomic radius slightly decreases. Also the nucleur charge or the proton number increases causing a greater nucleur charge and attraction forces between the nucleus and the valence electrons. So more energy is required
The above explanation is enough to score marks. However, this is the general trend and so there are some exception you need to know
Exceptions to the rule
The exception are only for group 2,3 and group 5,6 elements
As you guessed it, group 3 elements has a slightly lower ionisation energies than the group 2 elements. Why? We will see the answer below
The electrons of group 3 elements is added to the P subshell instead of the S orbital. So due to slight increase in atomic radius and shielding effect being more significant than the increase in nucleur charge. The effective nucleur charge decreases and so lower attractive force
If you are answering a question, ensure that you mention the principal quantum shells of the Subshells you are talking about. For example, 3P and 3S. Also remember to use the word "significant" in your answer as it is very important
Now we will go to the group 5, 6 exception
The group 6 has a lower ionisation energy compared to group 5 elements. The reason is a bit different and it includes the 4th factor
For group 6 elements, the electron is paired with another electron whereas for group 5 elements the p subshell is half filled with individual electrons. So due to the spin-pair repulsion of the electrons, it reduces the effective nucleur charge or attraction forces and so less energy is required
This idea is very important, that is why remember that group 5 elements have a half filled P subshell
This pattern works for almost across any period. So that's why it is part of the periodicity notes!
Trends down the group
To simply put it the ionisation energies decrease down the group. Why? We will see the answer
The increase in atomic radius and the increase in shielding effect due to increase in inner core electrons is more significant than the increase in nucleur charge or proton number. So the effective nucleur charge and the attraction forces between the electrons and the nucleus decreases and so the ionisation energy decreases
It is that simple and that's all you need to know! Make sure you use the word significant!
Identifying the element using Tabulated and Graphical data
For you to identify the element using data related to ionisation energies you need to look at the Successive ionisation energies of the element
The Successive ionisations of an elements means the series of ionisation energies of a particular element. For example the 1st, 2nd 3rd 4th...ionisation energies
The total number of Successive ionisation energies of an element is equal to the proton number of the element. This is because the electrons must equal to the number of protons. So you can indentify the element using this only
The Successive ionisation energies could be in the form of graphical data(graphs) or tabulated data
Tabulated Data
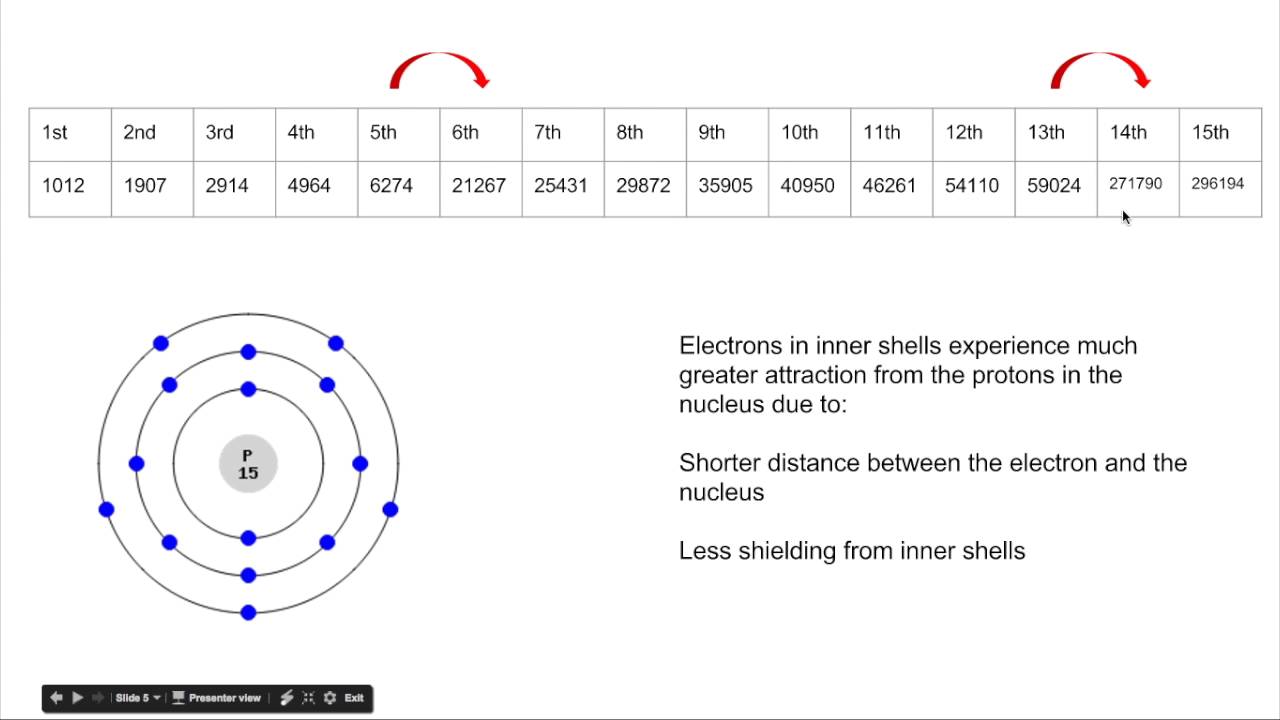
Usually they will not give you all the ionisation energies of an element but the first 8. As you can see the ionisation energy does increase for every step. This is because, when we remove electrons from the outer shell there is less repulsion force within the remaining electrons and so greater ionisation energy is needed
But sometimes, there will be a change or increase in ionisation energy which is the greatest! In this case, we need to understand that the largest change in ionisation energies is caused due to removing an electron from an inner shell(closer shell) so the ionisation energy is larger than normal
After finding this, we can find the number of electron in the outer most shell and predict its group number. The period can not be predicted and so we can not predict the element
Always make sure you find the GREATEST change in ionisation in the set of tabulated data
Graphical Data
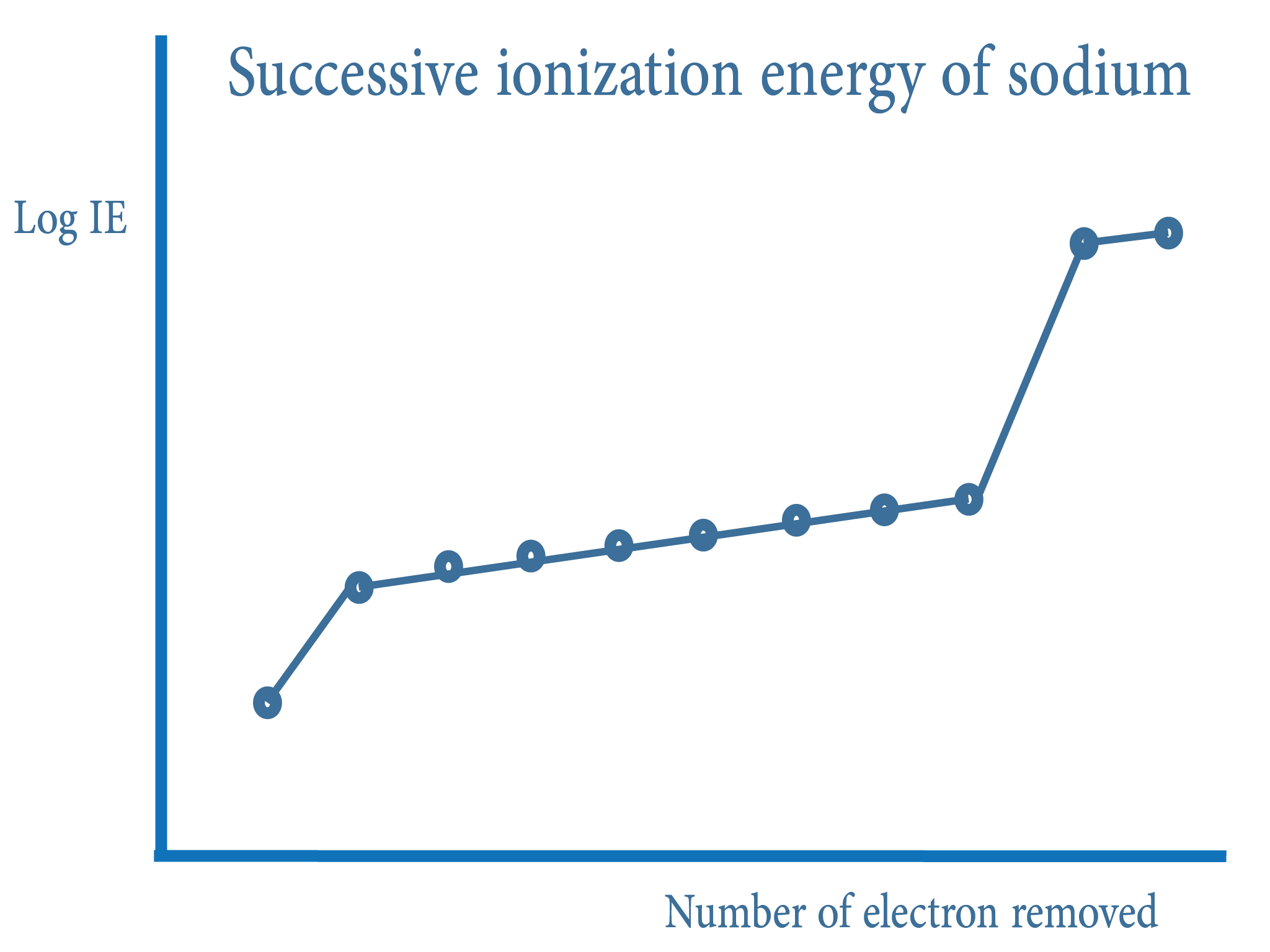
Graphical data can allow us to predict the period number and also the group number. In a graph, we can see places where there is a large increase in ionisation energies. This is because electrons are removed from a shell closer to the nucleus
The graph also has layers which contains very similar ionisation energies. The number of layers tells us the period number
Identify at which ionisation energy the first jump is being made. For example, if the jump is made at the 5th ionisation energy, it tells us there are 4 electrons in the outer most layer
By looking at these data, we can predict the element. We can also do this by looking at the total number of successful ionisation energies of an element
Atomic Radius
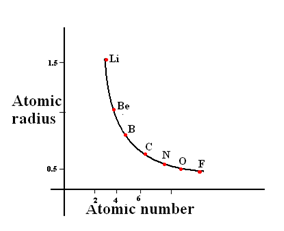
Atomic radius is a periodicity section but, we will still discuss it as it is related to ionisation energies
All you need to know that across a period, the atomic radius of elements decreases. Why? This is because, due to the increase in nucleur charge and shielding effect is more or less constant, there is greater attraction forces between the valence electrons and the nucleus and so greater pull. So the radius decreases
You will need to remember this graph and this is similar for any period
Exams question by asking to compare the atomic radius of 2 elements. For example, which one is larger in size, Na or Al. Even though it is tempting to think that because Al has more subshells, the atomic radius of Aluminium is less than Sodium. So Sodium is larger in size
Ionic Radius
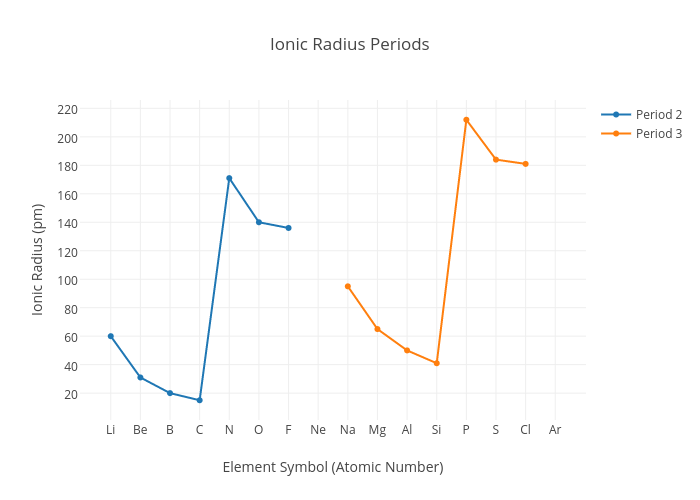
When comparing Ionic Radius, it is a bit different. Usually when elements form ions, they form the most stable electronic configuration
The Ionic radius of elements across a period also decreases but the anions have a larger radius than the cations. Remember the graph!
This is because, the anion has an extra principle quantum shell to hold more electrons and also there is greater shielding effect from inner core electrons
But for both Cations and Anions the Ionic radius decreases because the nucleur charge increases and the shielding effect is constant and so greater attraction and pull
Comparison between Ionic and Atomic Radius
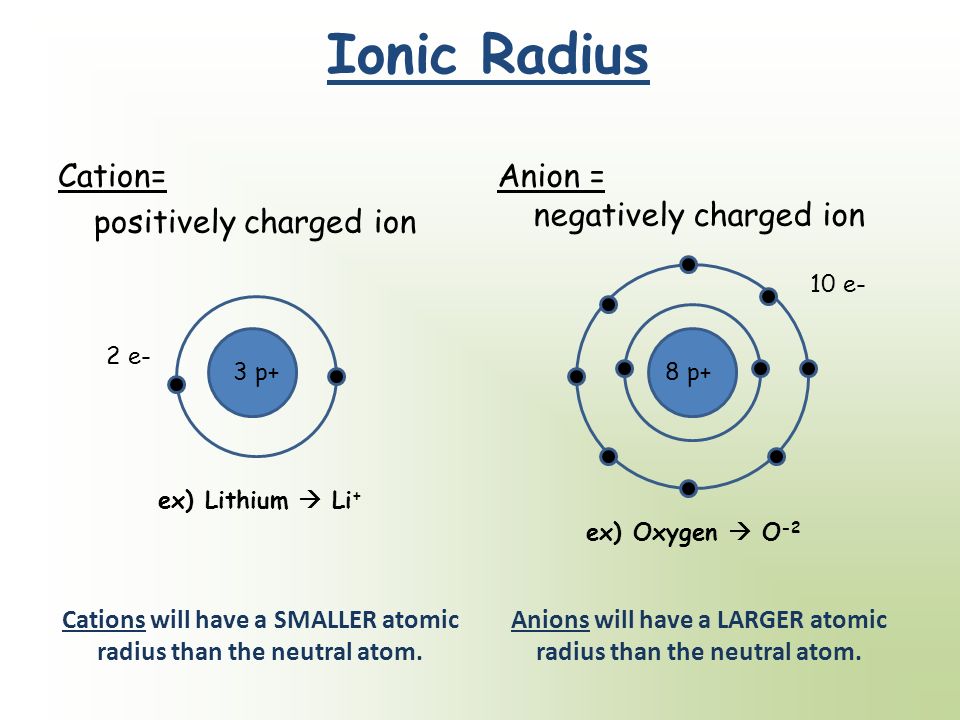
When we compare the Ionic or the atomic radius of a particular element, you need to consider whether it is a Anion or a cation!
Simply the cations has a smaller radius than the corresponding atom. Why? This is because, the cation has less electrons than the atom and so there is less repulsion force between electrons causing a greater EFFECTIVE nucleur charge on the valence electrons
The Anions has a larger radius than the corresponding atoms because, the anions have more electrons and so greater repulsion forces. This leads to a lower EFFECTIVE Nucleur charge on valence electrons so the radius is more larger
That's all you need to know. Please use the explanations above as they are from the markshemes!
