Chemistry
Introduction Moles Empirical Formulas & More Atomic Properties Ionisation Energies Bonding Intermolecular Forces States of Matter & Ideal Gases Giant Covalent & Ionic Structures Enthalpy Change Hess's Law Reaction Rate Equilibrium Redox Reactions Periodicity Group 2 Elements Group 7 Elements Nitrogen & SulfurOrganic Chemistry
Introduction to Organic Chemistry Organic Reactions Alkanes Alkenes Halogenoalkanes Alcohols & Carboxylic acids Aldehydes & Ketones PolymerisationMore
Reference Chapter QuestionsNitrogen & Sulfur
We will talk about some reactions, properties and uses of each element
Nitrogen
Nitrogen is a very inert gas and it is very unreactive. In fact, it is used as an inert gas to store and preserve products
The reason why nitrogen is very unreactive is because due to the presence of the triple bond in N2. So this requires a lot of energy to break the bond and so it has a very high activation energy. Also as the nitrogen molecule is non polar it is attacked less.
The air is 78% of Nitrogen and so Nitrogen can be obtained by fractional distillation of liquid air. Most reactions of nitrogen are endothermic and so we will see them below
Reactions of Nitrogen
Here are a few reactions of nitrogen
There are two situations in which oxides of nitrogen can be formed. It can be made from lightning activity because it gives sufficient energy to break the triple bond in N2
N2 + 2O2 → 2NO2
It can also be produced by internal combustion in engines, where nitrogen and oxygen in air react due to high temperature to form oxides of nitrogen
Oxides of nitrogen can be useful as it does fertilise the soil by adding nitrate ions, however, to much can cause acid rain and photochemical smog. To avoid this, we use a catalytic convertor, which will be discussed later
When a metal reacts with nitrogen under high temperatures a nitride is formed which is highly unstable
3Mg + N2 → Mg3N2
One of the most famous reactions used is the formation of ammonia
N2 + 3H2 ⇋ 2NH3
Its good to refer the equilibrium section to understand how different factors will affect the yield and equilibrium. We will state the conditions for Haber process
Catalyst: Iron filling
Temperature: 450°C
Pressure: 200atm
Another point to remember is that the ammonia has a higher melting point than nitrogen or hydrogen. so it can be obtained by fractional distillation by lowering the temperature
Ammonia
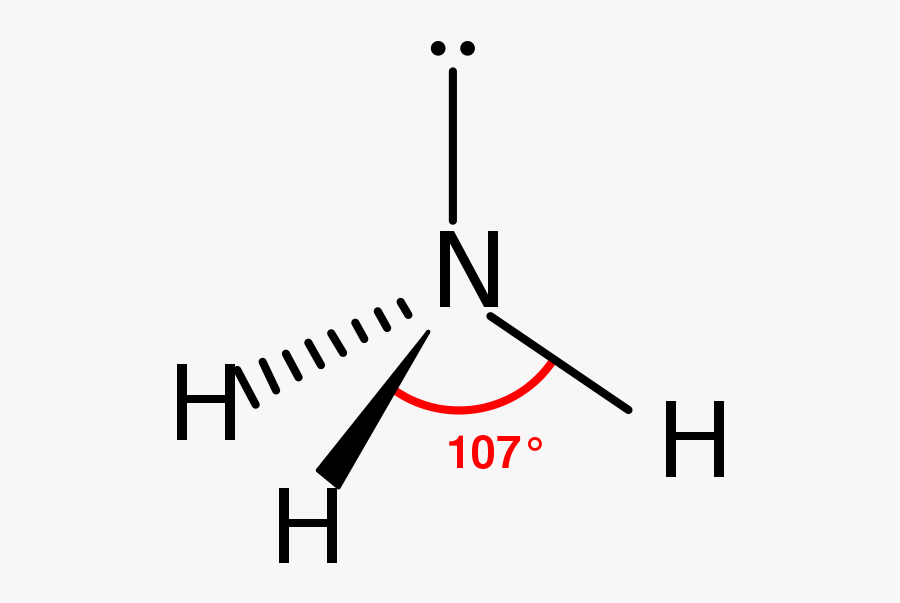
This is a structure of ammonia with a bond angle of 107° ( triangonal pyramidal shape )
Ammonia acts as a weak base due to presence of a lone pair. The lone pair accepts a proton to form the ammonium ion
Reactions of ammonia
There are some reactions of ammonia you need to know:
Ammonia acts as an base and so reacts with acids to form an ammonium salt
NH3 + HCl → NH4Cl
Ammonium salts reacts with bases when heated to form ammonia gas
NH4Cl + NaOH → NH3 + NaCl
This is used as a test for the presence of ammonium cations
A complex ion is formed known as a complex ligand
During this reaction the nitrogen does not oxidise at all and the nitrogen forms a dative bonding with the silver cation
Uses of ammonia
Ammonia is
- Used to make fertiliser or can be used as a fertiliser
- Used to produce nitric acid
- Used in household detergents
- Used to make nylon
Production of Nitric acid
This is not important but it is good to know. This process is called ostwald process
NH3 + O2 → NO + H2O
NO + O2 → NO2
NO2 + H2O → HNO3
There are many uses of nitric acid. Nitric acid can be used to make nylon and explosives and fertilizers
Ammonia as a fertiliser
Ammonia can be used directly as a fertilisers or it can be reacted to form salts which can be used as a fertilizer. For example, ammonia can be reacted with phosphoric acid to make ammonium phosphate
Fertilizer must contain these:
1. Nitrogen - This could be included in Nitrates or ammonia
2. Phosphate
3. Potassium
That's why we call these fertilizers, NPK fertilisers which includes all of the required nutrient that the plant needs
However, if excess fertilizers are used then this could lead to problems such as eutrophication. When fertilisers are sprayed in excess then this could lead to nutrients leeching from the soil to the lakes which may cause abnormal growth of algae in the water. This will then block the sunlight, and make the lower plants die. This will increase the number of decomposers in the water and reduce the oxygen concentration
Catalytic convertors
Due to the high temperature, Nitrogen oxide ( NO ) is formed in the engine. Also Carbon monoxide will be produced due to incomplete combustion of fossil fuels
NO and CO are pollutants and a very harmful to the enviroment. NO can cause acid rain and photochemical smog and CO is poisonous and toxic to living things as it reduces oxygen flow in blood
| Name | Hazards |
|---|---|
| VOCs or Unburnt Hydrocarbons | Causes cancer and smog |
| NO | Causes acid rain and smog |
| CO | Toxic, binds with blood to restrict oxygen flow |
| CO2 | Global warming |
These must be coverted to less harmful gases and so we use a catalytic convertor
Due to high temperature the NO reduces to N2 and the CO oxidises to CO2. This will still release carbon dioxide which will increase global warming. The equation can be simplified to:
2CO + 2NO → N2 + CO2
The catalytic has a honey comb shape to increase the rate of the reaction and to make sure that a high yield of polluntants are filtered. It uses platinum as its catalyst to speed up the reaction
Also unburnt hydrocarbons can also oxidise to form Carbon dioxide and water. This is required because, unburnt hydrocarbons or VOCs are cancerous substances and can cause smog
CxHy + O2 → CO2 + H2O
Disadvantages of Catalytic Convertor
1. Catalytic convertors are very expensive as they require precious platinum
2. Catalytic convertors only work when it reaches a high temperature so it is releases harmful gases while it reaches its optimum temperature
3. They may get clogged or inefficient over time so it must be replaced
4. Does not prevent Global warming
Sulfur
Sulfur is a yellow solid at room temperature and it exists in the form of S8. Sulfur has induced dipole forces between the molecules so it has a relatively low melting point
Sulfur can be produced from many sources, such as combustion of fuels containing sulfur impurities or burning sulfur ores or even from volcano activity
Sulfur has many uses such as:
Sulfur dioxide is a powerful reducing agent and it is mainly used as reductant to preserve food and kill bacteria. It is also used to form sulfuric acid
This process is known as contact process and sulfur is used to make sulfuric acid
Here are the step and equations related to contact process
S + O2 → SO2
The sulfur reacts or oxidises to form Sulfur dioxide
2S + 3O2 ⇋ 2SO3
Sulfur dioxide oxidises further in oxygen to form sulfur trioxide. For this there are some conditions you have to know
Catalyst: Vanadium(V) Oxide
Temperature: 450°C
Pressure: 1 atm
Then the sulfur trioxide is dissolved in concentrated sulfuric acid. They are not allowed to be dissolved directly in water as this will cause acid mist because the reaction is highly exothermic. So instead we dissolve it in sulfuric acid to get oleum
2SO3 + H2SO4 → H2S2O7
Then oleum is dilluted in water to form sulfuric acid
H2S2O7 + H2O → H2SO4
Sulfuric acid is a powerful oxidising agent and also a dehydrating substance and so it can be used for many purposes such as:
1. Fertilisers
2. Oven Cleaner
3. Tanning leather
4. Solvent & dyes & paints
Sulfuric acid is a powerful dehydrating chemical that can be used to dehydrate sugar and even alcohols to corresponding alkenes. This is more of an organic chemistry topic so it will be discussed later
Acid rain
Acid is caused by both sulfur and nitrogen but mostly sulfur. Sulfur dioxide and nitrogen dioxide can also produce acids when dissolved in water but this is not how acid rain is mainly formed. Below are the steps:
Sulfur dioxide produced from engines or volcanos are released to the air
NO from engines are released to the air and oxidises to form NO2
NO + O2 → NO2
The NO2 reacts with SO2 to form SO3. In other words, sulfur dioxide is oxidised by NO2
NO2 + SO2 → SO3 + NO
As we can see, NO is formed because it has been reduced. Then the SO3 dissolves in water to form Sulfuric acid
SO3 +H2O → H2SO4
Sulfuric acid is a strong acid so this is how mainly acid rain is formed. There is another step you need to know
The NO produced then oxidises to form NO2 again so it can attack another SO2. This is like a chain reaction and that is why we say that NO is a regenerating catalyst.
NO + O2 → NO2
Acid rain does cause many problems so we will see them below:
Damages caused by acid rain
There are so many so we will explain each point in depth:
Acid rain is an acid so it reacts with the nutrients in the soil and make them soluble. When it rains the soluble nutrients are washed away
This is done by lowering the pH of lakes or by increasing the Al ion concentration in water (caused by leeching). Al3+ ions are toxic to fish and kills them
As limestone is calcium carbonate it reacts with acids and erodes away. Also bridges are corroded by acid rain and lose their strength over time
